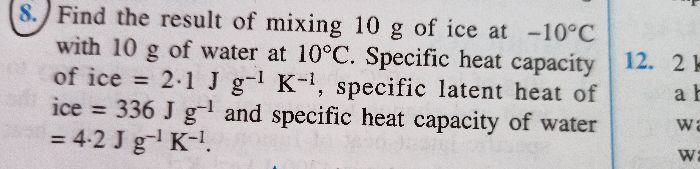ICSE Class 10 Answered
IF A HEATER WITH POWER 1000W GIVES 3.6*10^6 joules OF HEAT ENERGY IN ONE HOUR.WHAT WILL BE THE TEMPERATURE RAISED IN THE 50KG OF WATER TEMPERATURE 30 DEGREE CELCIUS BY THAT HEAT.
Asked by anish.kunwar.123 | 17 Jul, 2018, 21:11: PM
Heat required to raise the temperature of water is Q= 3.6×106 J.
Mass of water = 50 kg
We know that Q = m×c×Δt ... (1)
Rise in temperature Δt= tf - ti
A the temperature of water is 30°C,
Δt= tf - 30
Specific heat of water is 4200J/kg/°C.
From (1),
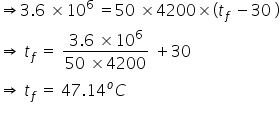
temperature of water raised due to this amount of energy is ( tf)= 47.14°C
→ Δt = 47.14°C - 30°C = 17.14°C
Thus, the rise in temperature is 17.14°C
Answered by Shiwani Sawant | 18 Jul, 2018, 12:21: PM
Concept Videos
ICSE 10 - Physics
Asked by chitrachongdar07 | 08 Dec, 2020, 10:55: AM
ICSE 10 - Physics
Asked by nilesh.dhote74 | 13 Jul, 2020, 12:38: PM
ICSE 10 - Physics
Asked by nilesh.dhote74 | 10 Jun, 2020, 15:00: PM
ICSE 10 - Physics
Asked by Himadri | 16 Apr, 2020, 14:21: PM
ICSE 10 - Physics
Asked by arpitt682 | 18 Jan, 2020, 20:11: PM
ICSE 10 - Physics
Asked by om.chaudhari1673 | 23 Jan, 2019, 16:37: PM
ICSE 10 - Physics
Asked by dhruuvsinghc123 | 04 Jan, 2019, 19:41: PM
ICSE 10 - Physics
Asked by dhruvilpatel020 | 26 Jul, 2018, 11:24: AM
ICSE 10 - Physics
Asked by anish.kunwar.123 | 17 Jul, 2018, 21:11: PM