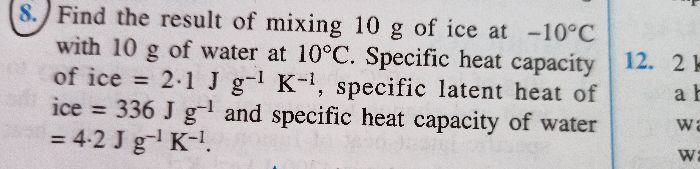ICSE Class 10 Answered
Why inorder to measure specific heat capacity we consider unit mass only and why is it not dependent on mass?
Asked by Himadri | 16 Apr, 2020, 02:21: PM
Specific heat capacity of a substance is the amount heat required for unit mass of substance to raise the temperature by 1º C .

Therefore,

It is just that we need to know the quantity of required heat energy per unit mass to calculate the specific heat.
It does not matter what is the mass of the substance, the specific heat capacity for that substance will remain same. Hence, specific heat capacity is independent of mass.
For example, if we have 100g of water in one beaker and 200 g of water in another the specific heat capacity of water will remain same which
is 4200 J kg-1 °C-1 = 4.2 J g-1 °C-1 (Remember that the specific heat capaity is intensive property of the substance)
Specific heat capacity of substance depends on the material of the substance
The specific heat capacity of the particular substance remains same and it does not depend on the mass of the substance.
It is the energy required to raise the temperature of unit mass of substance by 1ºC
-------------------------------------------------------------------------------------------------------------------------------------------------------------
However, if it is asked whether the heat capacity depends upon the mass, then the answer is 'Yes'
Heat capacity depends upon the mass of the substance.
There is difference between specific heat and heat capacity.
The heat capacity of a body is the amount of heat energy required to raise its temperature by 1°C or 1 K.
Answered by Shiwani Sawant | 16 Apr, 2020, 03:30: PM
Concept Videos
ICSE 10 - Physics
Asked by chitrachongdar07 | 08 Dec, 2020, 10:55: AM
ICSE 10 - Physics
Asked by nilesh.dhote74 | 13 Jul, 2020, 12:38: PM
ICSE 10 - Physics
Asked by nilesh.dhote74 | 10 Jun, 2020, 03:00: PM
ICSE 10 - Physics
Asked by Himadri | 16 Apr, 2020, 02:21: PM
ICSE 10 - Physics
Asked by arpitt682 | 18 Jan, 2020, 08:11: PM
ICSE 10 - Physics
Asked by om.chaudhari1673 | 23 Jan, 2019, 04:37: PM
ICSE 10 - Physics
Asked by dhruuvsinghc123 | 04 Jan, 2019, 07:41: PM
ICSE 10 - Physics
Asked by dhruvilpatel020 | 26 Jul, 2018, 11:24: AM
ICSE 10 - Physics
Asked by anish.kunwar.123 | 17 Jul, 2018, 09:11: PM