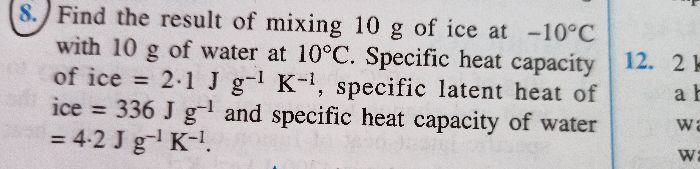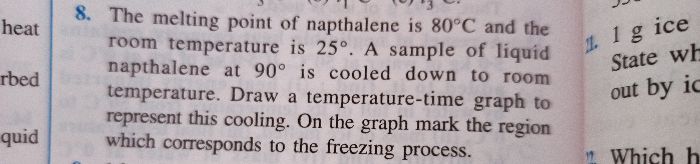ICSE Class 10 Answered
A Calorimeter of mass 50 grams and specific heat capacity 0.42 J/ g ? contains some mass of water at 20 ?. A metal piece of mass 20 grams at 100? is dropped into the calorimeter. After stirring, the final temperature is found to be 22?. Find the mass of water used in the calorimeter.
(Specific Heat capacity of metal piece: 0.3 J/g ?
Specific Heat capacity of water: 4.2 J/g ?)
Asked by om.chaudhari1673 | 23 Jan, 2019, 16:37: PM
Solution is attached.

Answered by Ankit K | 24 Jan, 2019, 11:43: AM
Concept Videos
ICSE 10 - Physics
Asked by chitrachongdar07 | 08 Dec, 2020, 10:55: AM
ICSE 10 - Physics
Asked by nilesh.dhote74 | 13 Jul, 2020, 12:38: PM
ICSE 10 - Physics
Asked by nilesh.dhote74 | 10 Jun, 2020, 15:00: PM
ICSE 10 - Physics
Asked by Himadri | 16 Apr, 2020, 14:21: PM
ICSE 10 - Physics
Asked by arpitt682 | 18 Jan, 2020, 20:11: PM
ICSE 10 - Physics
Asked by om.chaudhari1673 | 23 Jan, 2019, 16:37: PM
ICSE 10 - Physics
Asked by dhruuvsinghc123 | 04 Jan, 2019, 19:41: PM
ICSE 10 - Physics
Asked by dhruvilpatel020 | 26 Jul, 2018, 11:24: AM
ICSE 10 - Physics
Asked by anish.kunwar.123 | 17 Jul, 2018, 21:11: PM