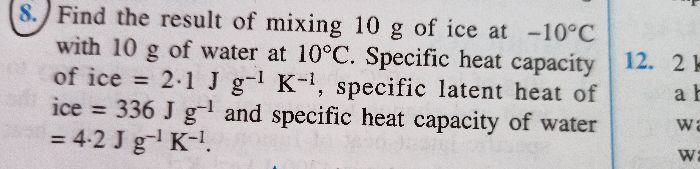ICSE Class 10 Answered
In our book, L=Q/m and Q=mc?t are given then why and how have you written Q=mc?t+mL?? In calorimetry, Ex B numerical no. 1
Asked by chaitalisen977 | 19 May, 2020, 15:32: PM
While solving such kind of problems based on change of phase of substance (Like in this case the ice (solid state) is converting to water(liquid state)) we have to consider the amount of heat energy required to raise the temperature of water which is at temperature 0°C to 50º C (as per the given problem) and also the heat energy required or exchanged while change of phase
Hence, as per the numerical,
Amount of heat energy required by 10g (0.01kg) of water at 0oC to raise its temperature by 50oC, Q1 = mΔct =0.01 X 4200 X 50=2100J
and
Amount of heat required to change the phase from ice to water, Q2 = mL
Hence,
Q = Q1 + Q2
Thus,
Q = mΔct + mL
Solve more problems based on this concept. This will help you to understand the concept easily.
Answered by Shiwani Sawant | 19 May, 2020, 17:44: PM
Concept Videos
ICSE 10 - Physics
Asked by imunilu786 | 29 Oct, 2023, 14:35: PM
ICSE 10 - Physics
Asked by surendrakumarclw | 20 Mar, 2021, 18:16: PM
ICSE 10 - Physics
Asked by anuradhastudent12 | 27 Feb, 2021, 20:33: PM
ICSE 10 - Physics
Asked by chitrachongdar07 | 08 Dec, 2020, 10:55: AM
ICSE 10 - Physics
Asked by charan3802 | 23 Oct, 2020, 10:29: AM
ICSE 10 - Physics
Asked by khushalkumarsk | 17 Aug, 2020, 12:37: PM
ICSE 10 - Physics
Asked by nilesh.dhote74 | 13 Jul, 2020, 12:38: PM
ICSE 10 - Physics
Asked by abhinabasarkar.abhinaba | 17 Jun, 2020, 21:24: PM
ICSE 10 - Physics
Asked by nilesh.dhote74 | 10 Jun, 2020, 15:00: PM
ICSE 10 - Physics
Asked by chaitalisen977 | 19 May, 2020, 15:32: PM