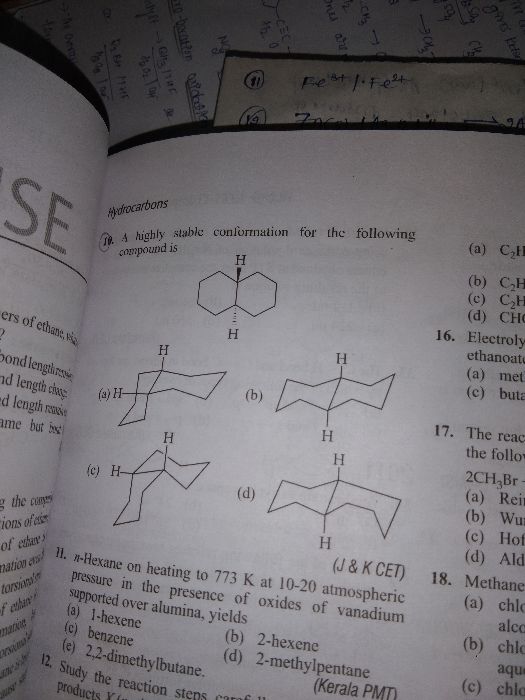
CBSE Class 11-science Answered
In catalytic cracking -
C2H6 ---> C2H4 + H2
In this reaction C2H6 is heated 750*C-1000*C.
Then why it does not convert into 2C + 3H2 ? as CH4 convert into C + 2H2 .
Asked by | 18 Feb, 2014, 07:59: PM
The alkanes , on heating under high temperature or in the presence of catalyst in absence of air are broken down into lower alkanes,alkenes and hydrogen.
Methane on cracking breaks down into ethyne and hydrogen.
Ethane on cracking breaks down into ethene and hydrogen.
Answered by | 19 Feb, 2014, 12:24: PM
Concept Videos
CBSE 11-science - Chemistry
Asked by kirithshiv | 24 Feb, 2024, 12:12: PM
CBSE 11-science - Chemistry
Asked by shivanij4734 | 16 Dec, 2023, 08:30: PM
CBSE 11-science - Chemistry
Asked by maibamjohnny89 | 15 Jan, 2022, 09:38: PM
CBSE 11-science - Chemistry
Asked by archu312004 | 07 Feb, 2021, 10:21: PM
CBSE 11-science - Chemistry
Asked by dubeyanubhav65 | 18 Jan, 2021, 09:52: PM
CBSE 11-science - Chemistry
Asked by shahsaqlain107 | 30 Nov, 2020, 01:10: PM
CBSE 11-science - Chemistry
Asked by ghastipratiksha | 11 Jul, 2020, 08:14: PM
CBSE 11-science - Chemistry
Asked by guptaserendri | 01 Jul, 2020, 03:58: PM
CBSE 11-science - Chemistry
Asked by Manpreetsingh669933 | 13 Apr, 2020, 01:39: PM
CBSE 11-science - Chemistry
Asked by bittutiwary1234 | 23 Feb, 2020, 12:15: AM