CBSE Class 9 Answered
In a reaction 5.3gm of sodium carbonate reacted with 6gm of ethanoic acid , the products were 2.2gm of carbon dioxide, 0.9gm of water and 8.2 gm of sodium ethanoic. Show that , these observations are in agreement with law of conservation of mass.
Asked by lopamudrabasak1996 | 11 Aug, 2019, 00:51: AM
Reaction of sodium carbonate reacted with ethanoic acid is,
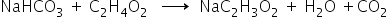
Mass of sodium carbonate = 5.3 gm
Mass of ethanoic acid = 6 gm
Mass of Sodium ethanoate = 8.2 gm
Mass of carbon dioxide = 2.2 gm
Mass of water = 0.9 gm
Total mass of reactant = (5.3 + 6)
=11.3 gm
Total mass of products =( 8.2+2.2+0.9 )
= 11.3 gm
Total mass reactant = Total mass of products.
Therefore the observations are in agreement with law of conservation of mass.
Answered by Varsha | 12 Aug, 2019, 09:54: AM
Application Videos
Concept Videos
CBSE 9 - Chemistry
Asked by Niharikadhamija13 | 25 Aug, 2020, 17:17: PM
CBSE 9 - Chemistry
Asked by haritchahar | 25 Jul, 2020, 11:55: AM
CBSE 9 - Chemistry
Asked by yanagauswami00.tl | 17 Apr, 2020, 10:44: AM
CBSE 9 - Chemistry
Asked by harshilmodi74.tl | 16 Apr, 2020, 10:39: AM
CBSE 9 - Chemistry
Asked by harshilmodi74.tl | 16 Apr, 2020, 10:35: AM
CBSE 9 - Chemistry
Asked by rjinaaishu007 | 10 Feb, 2020, 19:05: PM
CBSE 9 - Chemistry
Asked by prakash.sanyasi | 09 Feb, 2020, 22:59: PM
CBSE 9 - Chemistry
Asked by kumaruditanshu27 | 21 Oct, 2019, 18:16: PM
CBSE 9 - Chemistry
Asked by guptarushil6 | 15 Oct, 2019, 22:41: PM
CBSE 9 - Chemistry
Asked by lopamudrabasak1996 | 11 Aug, 2019, 00:51: AM