CBSE Class 11-science Answered
How many nodal planes do the SIGMA Pz ABMO.have and How?
Asked by Shrivatsa | 25 Aug, 2019, 02:11: PM
There is one nodal plane in sigma pz antibonding orbital.
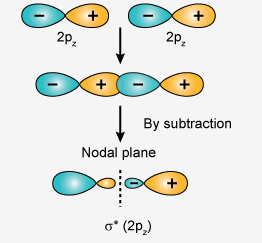
Dotted line is representing a nodal plane.
Answered by Ravi | 26 Aug, 2019, 12:34: PM
Concept Videos
CBSE 11-science - Chemistry
Asked by thesouro007 | 20 Mar, 2024, 06:05: AM
CBSE 11-science - Chemistry
Asked by shabnamaijaz83 | 19 Jun, 2022, 10:08: AM
CBSE 11-science - Chemistry
Asked by abnarsale | 31 Dec, 2021, 10:41: AM
CBSE 11-science - Chemistry
Asked by defence | 17 Feb, 2020, 05:09: PM
CBSE 11-science - Chemistry
Asked by mandriosa67 | 13 Feb, 2020, 03:03: PM
CBSE 11-science - Chemistry
Asked by Shrivatsa | 25 Aug, 2019, 02:11: PM
CBSE 11-science - Chemistry
Asked by sonkarshiva009 | 13 Mar, 2019, 05:47: PM
CBSE 11-science - Chemistry
Asked by vermaarti729 | 28 Feb, 2019, 08:28: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM



 4.IF7
5.
4.IF7
5. B.Explain anti bonding and bonding hybridised orbitals.
C._________on hydrolysis gives ethyne while ______ on hydrolysis gives methane.
D.Explain why the colour of Bayer's reagent gets discharged when treated with an alkene.
E.i) State and explain Le Chatelier’s principle. On the basis of this principle discuss the conditions for obtaining the maximum yield of SO3 in the following reaction. 2SO2(g)+ O2(g)⇌2SO3(g); ∆𝐻= - 42k.cal.(ii) Calculate the pH value of 0.01M CH3 COOH if it is 5% dissociated.
B.Explain anti bonding and bonding hybridised orbitals.
C._________on hydrolysis gives ethyne while ______ on hydrolysis gives methane.
D.Explain why the colour of Bayer's reagent gets discharged when treated with an alkene.
E.i) State and explain Le Chatelier’s principle. On the basis of this principle discuss the conditions for obtaining the maximum yield of SO3 in the following reaction. 2SO2(g)+ O2(g)⇌2SO3(g); ∆𝐻= - 42k.cal.(ii) Calculate the pH value of 0.01M CH3 COOH if it is 5% dissociated.