CBSE Class 11-science Answered
How does the Second Law of Thermodynamics help in the working of a refrigerator?
Asked by Topperlearning User | 17 Apr, 2015, 14:29: PM
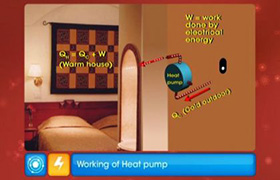
A way of stating the second law of thermodynamics is that work must be done to get heat to flow from a cold object to a hot object. In a refrigerator, there is a cycle that is carried on continuously. A liquid refrigerant substance vaporizes in the cooling coils inside the fridge. The fluid absorbs heat from its surroundings to vaporize. This cools the interior of the fridge. The gas thus formed is pumped to the exterior of the fridge where it is compressed into a liquid. Work is done on the gas to compress the gas, causing the gas to release heat. This heat is lost to the air surrounding the fridge. Hence, heat is moved from the inside of the fridge to the outside.
Answered by | 17 Apr, 2015, 16:29: PM
Concept Videos
CBSE 11-science - Physics
Asked by bbabichowdary | 02 Sep, 2020, 13:25: PM
CBSE 11-science - Physics
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM
CBSE 11-science - Physics
Asked by Topperlearning User | 17 Apr, 2015, 14:29: PM
CBSE 11-science - Physics
Asked by Topperlearning User | 17 Apr, 2015, 16:46: PM
CBSE 11-science - Physics
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM
CBSE 11-science - Physics
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM
CBSE 11-science - Physics
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM
CBSE 11-science - Physics
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM
CBSE 11-science - Physics
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM
CBSE 11-science - Physics
Asked by Topperlearning User | 17 Apr, 2015, 16:54: PM