CBSE Class 12-science Answered
how does arylhalide react with alcoholic ammmonia ?
Asked by krishna | 23 Nov, 2013, 09:40: AM
Alkyl halides react with alcoholic ammonia solution to give amines. In this reaction, alkyl halide undergoes nucleophilic substitution reaction.
RX + NH3 → R NH3 +X-
R NH3 +X- + NH3 → RNH2 + NH4 +X-
10 amine
RNH2 + RX → R2NH + RX → R3N + RX → R4N+X-
10 amine 20 amine 30 amine Quaternary ammonium salt
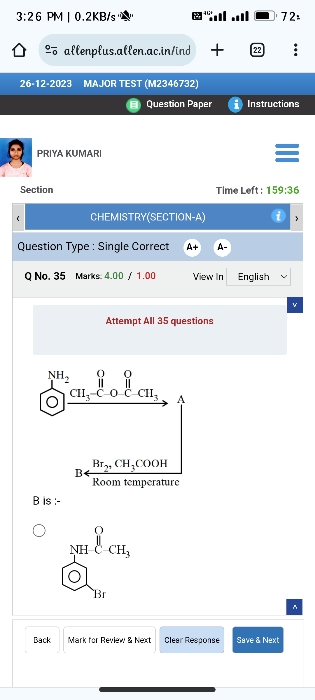
Aryl halides do not react with alcoholic ammonia solution because aryl halides are relatively less reactive than alkyl halides towards nucleophilic substitution reactions.
Answered by Karishma Kapoor | 25 Nov, 2013, 12:34: PM
Concept Videos
CBSE 12-science - Chemistry
Asked by hanihope27 | 01 Mar, 2024, 08:33: PM
CBSE 12-science - Chemistry
Asked by priyankapaliwal255 | 23 Sep, 2023, 05:46: AM
CBSE 12-science - Chemistry
Asked by shwetayaligar205 | 07 Jul, 2022, 08:13: PM
CBSE 12-science - Chemistry
Asked by kaziryan.05 | 06 Jul, 2021, 11:31: PM
CBSE 12-science - Chemistry
Asked by dhivagar25375 | 12 Aug, 2020, 08:34: PM
CBSE 12-science - Chemistry
Asked by danapalanandhan | 28 Jul, 2020, 11:48: AM
CBSE 12-science - Chemistry
Asked by sulaikhasulu393 | 27 May, 2020, 03:34: PM
CBSE 12-science - Chemistry
Asked by mufeedatvp2000 | 15 Apr, 2020, 01:35: PM