CBSE Class 12-science Answered
Half life of reaction : H2O2(aq)= H2O(l) + 1/2 O2 (g) is independent of initial concentration of H2O2 volume of O2 gas after 20 minute is 5L at 1 atm and 27degreeC and after completion of reaction is 50L. The rate constant is :
(1) 1/20 log 10 min ^-1
(2) 2.303/20 log 10 min ^-1
(3) 2.303/20 log 50/45 min ^-1
(4) 2.303/20 log 45/50 min ^-1
Asked by thakurpratyush5 | 14 Aug, 2015, 07:57: PM
The correct option is 3 as this reaction is first order reaction.
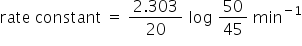
Answered by Prachi Sawant | 15 Aug, 2015, 11:01: PM
Concept Videos
CBSE 12-science - Chemistry
Asked by amitkumar.cis | 14 Jan, 2021, 12:40: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 28 Mar, 2014, 10:16: AM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 22 Jun, 2016, 09:20: AM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 22 Jun, 2016, 09:17: AM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 22 Jun, 2016, 09:18: AM





