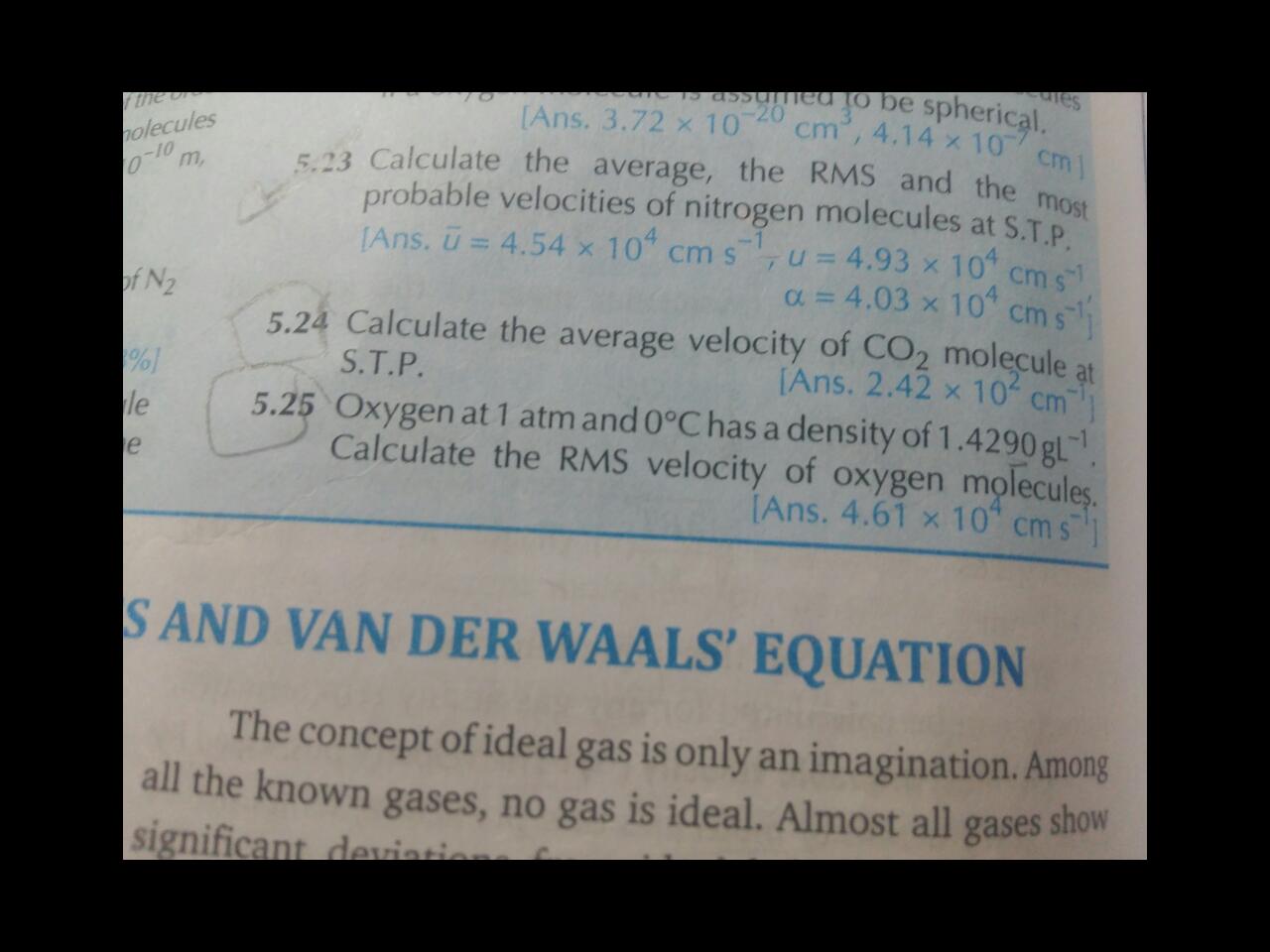CBSE Class 11-science Answered
Give two evidences with explanation of molecular motion of gases.
Asked by aiqbal86592 | 28 May, 2019, 12:20: PM
Random movement of dust particles-When dust particles colloids with air. It move srandomly. so this random motion explains the colision in molecular motion of gas present in air and it shows that gas particles are in constant random motion.
Diffusion of Ammonia and HCl gas-
We soak mcotton in ammonia and HCl and place at two different ends. For each cotton wool, chemicals evaopurate. These vapours move in random motion in air. Ammonia moves faster than HCl because it has lower mass.
Answered by Ravi | 28 May, 2019, 01:05: PM
Concept Videos
CBSE 11-science - Chemistry
Asked by khansuhana410 | 22 Dec, 2022, 11:48: AM
CBSE 11-science - Chemistry
Asked by aiqbal86592 | 28 May, 2019, 12:20: PM
CBSE 11-science - Chemistry
Asked by lovemaan5500 | 26 Jan, 2019, 12:57: PM
CBSE 11-science - Chemistry
Asked by nasankalagi04 | 17 Oct, 2018, 08:47: PM
CBSE 11-science - Chemistry
Asked by smanishkumar2002 | 04 Aug, 2018, 05:38: AM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 17 Jun, 2016, 12:02: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 21 Apr, 2015, 12:19: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 21 Apr, 2015, 12:21: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 21 Apr, 2015, 12:31: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 17 Jun, 2016, 12:01: PM