CBSE Class 11-science Answered
Give the value of P, V, and T at STP and NTP
Asked by adisuncheti | 26 Aug, 2021, 00:56: AM
Temperature and Pressure at STP is 273.15 K and 0.987 atm respectively.
Temperature and Pressure at NTP is 293.15 K and 1 atm resapectively.
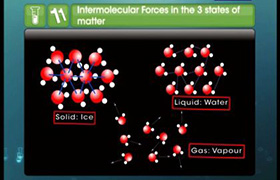
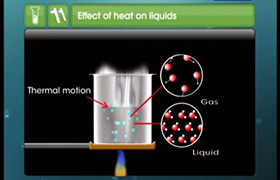
Voume depends on other factors but for ideal gas it is taken 22.4 litre in both cases.
Answered by Ravi | 26 Aug, 2021, 15:36: PM
Concept Videos
CBSE 11-science - Chemistry
Asked by luvs6482 | 27 Apr, 2024, 20:18: PM
CBSE 11-science - Chemistry
Asked by smanishkumar2002 | 04 Aug, 2018, 05:36: AM
CBSE 11-science - Chemistry
Asked by tps.mjmdr | 22 Jun, 2018, 18:36: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 20 Apr, 2015, 14:31: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 20 Apr, 2015, 14:33: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 20 Apr, 2015, 14:34: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 20 Apr, 2015, 14:36: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 17 Jun, 2016, 11:24: AM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 17 Jun, 2016, 11:23: AM