CBSE Class 12-science Answered
Give a note on the chemical reaction of lanthanoids.
Asked by Topperlearning User | 27 Jun, 2014, 03:42: PM
The earlier members of the series behave like calcium.
Ln3+(aq) + 3e– 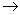 Ln(s)
Ln(s)
The Eo value for the above reaction ranges from –2.2 to –2.4 V. They show following types of reaction
Ln + Acid 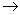 H2
H2
Ln + H2O 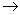 Ln(OH)3 + H2
Ln(OH)3 + H2
Ln + N 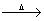 LnN
LnN
Ln + C 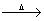 LnC2
LnC2
Ln + S 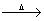 Ln2S3
Ln2S3
Answered by | 27 Jun, 2014, 05:42: PM
Concept Videos
CBSE 12-science - Chemistry
Asked by asikmostafamondal8 | 02 May, 2021, 09:08: AM
CBSE 12-science - Chemistry
Asked by ndevi1234 | 11 Mar, 2019, 05:30: AM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 06 Jun, 2016, 02:19: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 27 Jun, 2014, 02:42: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 27 Jun, 2014, 03:34: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM




