CBSE Class 12-science Answered
Explain with the help of example INDUCED CATALYST
Asked by pardeepkumar2281 | 31 Jul, 2018, 11:40: PM
When one reaction influences the rate of other reaction, which does not occur under ordinary conditions, then this phenomenon is induced catalysis.
Example:
Example:
Sodium sulphite solution oxidises in the air but sodium arsenite solution does not oxidise by passing air.
But when both solutions are mixed and the air is passed through the solution, then both the substances get oxidised.
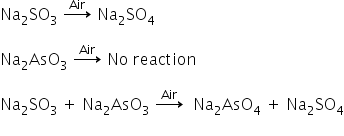
Answered by Varsha | 01 Aug, 2018, 11:14: AM
Concept Videos
CBSE 12-science - Chemistry
Asked by sivakapuganti1 | 26 Aug, 2020, 08:57: PM
CBSE 12-science - Chemistry
Asked by spoorthysaienelluri | 23 May, 2020, 11:07: AM
CBSE 12-science - Chemistry
Asked by vermahitesh124 | 12 May, 2020, 07:58: AM
CBSE 12-science - Chemistry
Asked by yogeshsulakh | 07 Feb, 2020, 09:28: AM
CBSE 12-science - Chemistry
Asked by tlb2bpartner | 21 Aug, 2019, 10:28: PM
CBSE 12-science - Chemistry
Asked by niharikapabba2605 | 07 Aug, 2018, 11:38: AM
CBSE 12-science - Chemistry
Asked by pardeepkumar2281 | 31 Jul, 2018, 11:40: PM
CBSE 12-science - Chemistry
Asked by pardeepkumar2281 | 31 Jul, 2018, 11:38: PM
CBSE 12-science - Chemistry
Asked by pardeepkumar2281 | 31 Jul, 2018, 11:36: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM