CBSE Class 12-science Answered
its cyclopropane, cyclopentane or cyclohexane and benzene Generally there are two types of carbenes; singlet or triplet carbenes. Singlet carbenes have a pair of electrons and an sp2 hybrid structure. Triplet carbenes have two unpaired electrons. They may be either sp2 hybrid or linear sp hybrid. Most carbenes have a nonlinear triplet ground state, except for those with nitrogen, oxygen, or sulfur atoms, and dihalocarbenes.Carbenes are called singlet or triplet depending on the electronic spins they possess. Triplet carbenes are paramagnetic and may be observed by electron spin resonance spectroscopy if they persist long enough. The total spin of singlet carbenes is zero while that of triplet carbenes is one (in units of 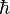 ). Bond angles are 125-140° for triplet methylene and 102° for singlet methylene (as determined by EPR). Triplet carbenes are generally stable in the gaseous state, while singlet carbenes occur more often in aqueous media Singlet and triplet carbenes do not demonstrate the same reactivity. Singlet carbenes generally participate in cheletropic reactions as either electrophiles or nucleophiles. Singlet carbene with its unfilled p-orbital should be electrophilic. Triplet carbenes should be considered to be diradicals, and participate in stepwise radical additions. Triplet carbenes have to go through an intermediate with two unpaired electrons whereas singlet carbene can react in a single concerted step. Addition of singlet carbenes to olefinic double bonds is more stereoselective than that of triplet carbenes. Addition reactions with alkenes can be used to determine whether the singlet or triplet carbene is involved.
). Bond angles are 125-140° for triplet methylene and 102° for singlet methylene (as determined by EPR). Triplet carbenes are generally stable in the gaseous state, while singlet carbenes occur more often in aqueous media Singlet and triplet carbenes do not demonstrate the same reactivity. Singlet carbenes generally participate in cheletropic reactions as either electrophiles or nucleophiles. Singlet carbene with its unfilled p-orbital should be electrophilic. Triplet carbenes should be considered to be diradicals, and participate in stepwise radical additions. Triplet carbenes have to go through an intermediate with two unpaired electrons whereas singlet carbene can react in a single concerted step. Addition of singlet carbenes to olefinic double bonds is more stereoselective than that of triplet carbenes. Addition reactions with alkenes can be used to determine whether the singlet or triplet carbene is involved.
Intramolecular insertion reactions present new synthetic solutions. Generally, rigid structures favor such insertions to happen. When an intramolecular insertion is possible, no intermolecular insertions are seen. In flexible structures, five-membered ring formation is preferred to six-membered ring formation. Both inter- and intramolecular insertions are amendable to asymmetric induction by choosing chiral ligands on metal centers
The reaction of Fischer carbene complexes that has grown to greatest prominence in applications in organic synthesis is the benzannulation reaction. This is a truly amazing reaction that was discovered by Karl Heinz Dötz and involves the reaction of unsaturated complexes with alkynes to give 4-alkoxyphenols of the type 4 as the primary product of the reaction.The initial product of the reaction is 3, the corresponding arene chromium tricarbonyl complex of the phenol 4, but these typically are unstable to air such that workup in air and purification of the product on silica gel leads to the complete loss of the metal.The phenol 4 is the result of the assembly of the alkyne, the carbene ligand and a carbon monoxide ligand in the coordination sphere of the metal. That this highly orchestrated process occurs under neutral conditions and near ambient temperatures (45 oC) to typically produce high yields of the phenol products