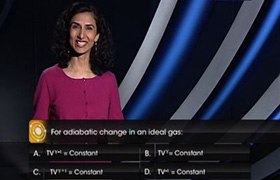
CBSE Class 11-science Answered
dd
Asked by mr.mkumar12 | 14 Jan, 2024, 13:34: PM
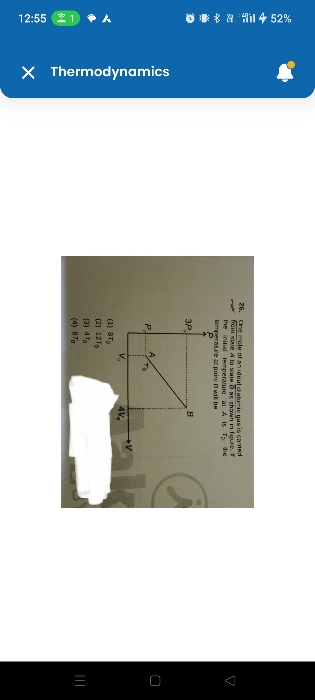
ideal gas equation is " P V = n R T " , where P is pressure , V is volume , n is number of moles ,
R is universal gas constant and T is temperature .
Hence we get . ( P V ) / T is constant at any state
Hence from known pressure and volume values at state A and B , we get
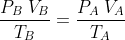
from above expression, we get
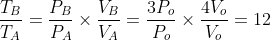
TB = 12 TA = 12 To
Answered by Thiyagarajan K | 14 Jan, 2024, 16:57: PM
Concept Videos
CBSE 11-science - Physics
Asked by shubham23302007 | 23 Jan, 2024, 22:24: PM
CBSE 11-science - Physics
Asked by s3043632 | 22 Jan, 2023, 18:45: PM
CBSE 11-science - Physics
Asked by govtsecschoolnayaganv051 | 14 Dec, 2018, 19:19: PM
CBSE 11-science - Physics
Asked by Topperlearning User | 17 Apr, 2015, 10:09: AM
CBSE 11-science - Physics
Asked by Topperlearning User | 17 Apr, 2015, 10:11: AM
CBSE 11-science - Physics
Asked by araima2001 | 17 Mar, 2017, 08:12: AM
CBSE 11-science - Physics
Asked by Kusum and Sanjeet | 13 Jul, 2015, 22:02: PM