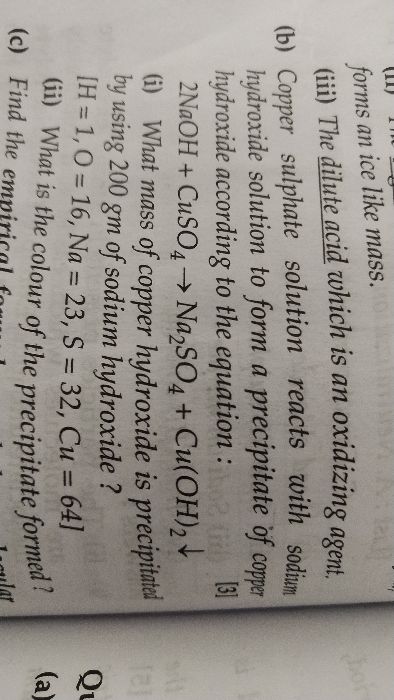ICSE Class 10 Answered
Calculate weight in gram, number of moles and number of molecules in 1120 cm^3 of N2(Nitrogen gas) at S.T.P. (Take Avogadro number 6 x 10^23).
Asked by dr_pradip27121972 | 25 Mar, 2018, 03:38: PM
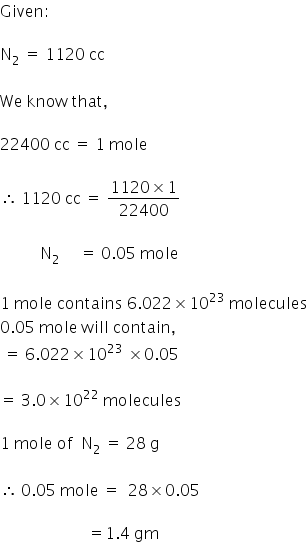
Weight of N2 = 1.4 gm
No.of molecules = 3 ×1022
Moles of N2 = 0.05 mol
Answered by Varsha | 26 Mar, 2018, 06:20: PM
Application Videos
Concept Videos
ICSE 10 - Chemistry
Asked by jrvedant208 | 05 Feb, 2024, 10:37: PM
ICSE 10 - Chemistry
Asked by rashikulkarni28 | 10 Jul, 2022, 10:13: PM
ICSE 10 - Chemistry
Asked by rashikulkarni28 | 25 Jun, 2022, 10:24: PM
ICSE 10 - Chemistry
Asked by palshivom72 | 14 Jul, 2020, 07:56: PM
ICSE 10 - Chemistry
Asked by jhabijay01 | 27 May, 2020, 12:20: PM
ICSE 10 - Chemistry
Asked by aashimegh | 04 Sep, 2019, 08:53: AM
ICSE 10 - Chemistry
Asked by aashimegh | 04 Sep, 2019, 08:37: AM
ICSE 10 - Chemistry
Asked by aashimegh | 28 Aug, 2019, 05:25: PM