CBSE Class 12-science Answered
calculate the molarity of solution prepared by mixing equal amount of 30% by weight of H2SO4(density 1.128g/ml) and 70% by weight of H2SO4(density 1.610 g/ml).
Asked by prernamehta | 13 Jul, 2011, 10:19: PM
30 % by weight solution of 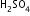 means 30g of
means 30g of 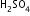 is dissolved in 100g of solution.
is dissolved in 100g of solution.
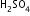 means 30g of
means 30g of 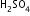 is dissolved in 100g of solution.
is dissolved in 100g of solution.Let volume of each solution mixed =100 ml
Therefore, total volume of solution = 200 ml
Wt. of 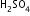 in 30 % solution =
in 30 % solution = 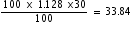 g
g
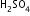 in 30 % solution =
in 30 % solution = 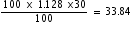 g
gWt. of  in 70 % solution =
in 70 % solution = 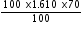 =112.7 g
=112.7 g
 in 70 % solution =
in 70 % solution = 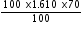 =112.7 g
=112.7 g Total wt. of 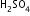 = 33.84 +112.7 =146.54 g
= 33.84 +112.7 =146.54 g
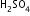 = 33.84 +112.7 =146.54 g
= 33.84 +112.7 =146.54 gNow, Molarity = 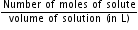
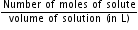
= 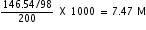
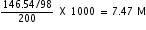
Answered by | 14 Jul, 2011, 10:00: AM
Concept Videos
CBSE 12-science - Chemistry
Asked by hannamaryphilip | 17 Apr, 2024, 11:20: PM
CBSE 12-science - Chemistry
Asked by sameerteli003 | 08 Apr, 2024, 11:48: PM
CBSE 12-science - Chemistry
Asked by rashmij34 | 27 Feb, 2024, 04:42: PM
CBSE 12-science - Chemistry
Asked by sagarmishra | 27 Feb, 2024, 04:01: PM
CBSE 12-science - Chemistry
Asked by kalandi.charan.407 | 08 Feb, 2024, 01:42: PM
CBSE 12-science - Chemistry
Asked by premkhare2006 | 24 Jan, 2024, 09:50: AM
CBSE 12-science - Chemistry
Asked by saritanohar22 | 13 Jan, 2024, 01:25: PM
CBSE 12-science - Chemistry
Asked by kaushikmisty07 | 31 Dec, 2023, 11:42: AM
CBSE 12-science - Chemistry
Asked by kamlesh.kumar.malee | 20 Dec, 2023, 06:59: AM
CBSE 12-science - Chemistry
Asked by varinder2149 | 10 Dec, 2023, 08:21: PM








