CBSE Class 11-science Answered
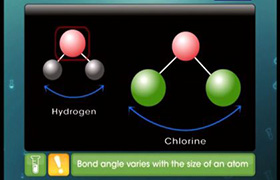
Bond order difference H2-H2+
and H2
Asked by abdulhamid02427 | 24 Dec, 2020, 11:51: AM
Bond Order-
H2 , 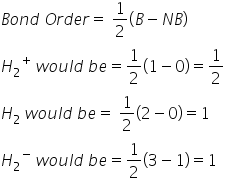 H2-
H2-
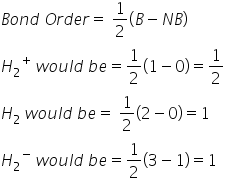 H2-
H2-
Answered by Ravi | 31 Dec, 2020, 19:15: PM
Concept Videos
CBSE 11-science - Chemistry
Asked by rushikeshpandav00 | 17 Jun, 2021, 13:43: PM
CBSE 11-science - Chemistry
Asked by mittalneetu345 | 12 Feb, 2021, 10:23: AM
CBSE 11-science - Chemistry
Asked by abdulhamid02427 | 24 Dec, 2020, 11:51: AM
CBSE 11-science - Chemistry
Asked by rayyan20151 | 09 Jan, 2020, 11:40: AM
CBSE 11-science - Chemistry
Asked by ABHILASHA | 07 Sep, 2019, 15:59: PM
CBSE 11-science - Chemistry
Asked by narayandhar1979 | 28 Oct, 2018, 10:25: AM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 08 Oct, 2014, 08:44: AM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 07 Oct, 2014, 17:14: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 08 Oct, 2014, 08:51: AM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM