CBSE Class 10 Answered
Sodium (2,8,1) has 1 electron more than a stable noble gas structure (2,8). If it gave away that electron it would become more stable.
Chlorine (2,8,7) has 1 electron short of a stable noble gas structure (2,8,8). If it could gain an electron from somewhere it too would become more stable.
The answer is obvious. If a sodium atom gives an electron to a chlorine atom, both become more stable.
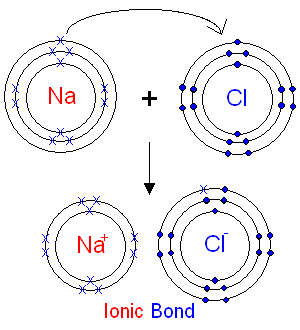
The sodium has lost an electron, so it no longer has equal numbers of electrons and protons. Because it has one more proton than electron, it has a charge of 1+. If electrons are lost from an atom, the number of protons exceeds the number of negatively charged electrons hence, positive ions called cations are formed.
The chlorine has gained an electron, so it now has one more electron than proton. It therefore has a charge of 1-. If electrons are gained by an atom, the number of negatively charged electrons exceeds the number of protons hence, negative ions called anions are formed.
The sodium ions and chloride ions being oppositely charged are held together by the strong electrostatic attractions
Since only one sodium atom is needed to provide the extra electron for one chlorine atom, so they combine together in 1:1 ratio. The formula is therefore NaCl.











