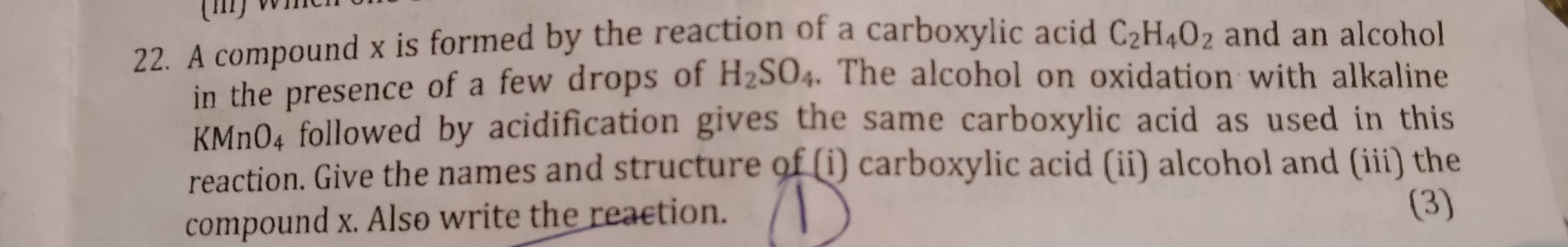CBSE Class 10 Answered
Answer the following questions-
(a) Describe a chemical test to distinguish between ethanol and ethanoic acid.
(b) Give reason for the following:
(i) Ethanol is used in the preparation of tincture iodine.
(ii) Ethanoic acid is used in the preservation of pickles.
(c) What is the effect of ethanoic acid on universal indicator?
Asked by Topperlearning User | 05 Feb, 2015, 12:53: PM
(a) NaHCO3 test - Ethanoic acid reacts with sodium hydrogen carbonate to give rise to sodium acetate, carbon dioxide and water. Whereas, ethanol does not produce carbon dioxide on reaction with sodium hydrogen carbonate.
CH3COOH + NaHCO3 → CH3COONa + H2O + CO2
(b)
(1) Ethanol is used in the preparation of tincture of iodine as it acts as a solvent.
(2) Ethanoic acid is used in the preservation of pickles because it kills bacteria.
(c) Dilute ethanoic acid turns universal indicator paper to orange, indicating that its pH is about 4. It is a weak acid.
Answered by | 05 Feb, 2015, 14:53: PM
Application Videos
Concept Videos
CBSE 10 - Chemistry
Asked by sneh | 27 Mar, 2020, 10:11: AM
CBSE 10 - Chemistry
Asked by pranjaliinamdar2004 | 29 Feb, 2020, 19:20: PM
CBSE 10 - Chemistry
Asked by sweetykhatri99254 | 27 Feb, 2020, 15:40: PM
CBSE 10 - Chemistry
Asked by priyanshiishu | 30 Jan, 2020, 10:39: AM
CBSE 10 - Chemistry
Asked by kamalnayansingh7 | 13 Jan, 2020, 08:35: AM
CBSE 10 - Chemistry
Asked by Deepak | 22 Dec, 2019, 23:20: PM
CBSE 10 - Chemistry
Asked by ritikraghuwanshi6986 | 16 Dec, 2019, 20:42: PM
CBSE 10 - Chemistry
Asked by vedantsagrawal23 | 05 Dec, 2019, 08:34: AM
CBSE 10 - Chemistry
Asked by aryasaxena2003 | 25 Jul, 2019, 17:35: PM
CBSE 10 - Chemistry
Asked by rushabhjain.avv | 21 Mar, 2019, 22:07: PM