CBSE Class 12-science Answered
An element X' (At. mass a 40 g mol-1) having f.c.c. structure, has unit cell edge length of 400 pm. Calculate the density of ‘X’ and the number of unit cells in 4 g of ‘X’. (NA = 6.022 x 1023 mol-1)
Asked by raul.chintakindi14 | 14 Mar, 2018, 11:44: AM
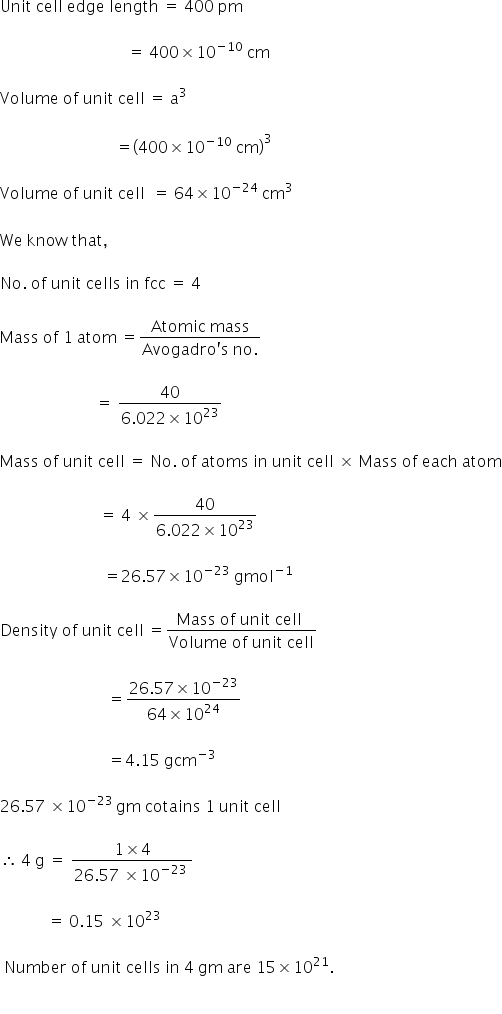
Answered by Varsha | 14 Mar, 2018, 02:44: PM
Concept Videos
CBSE 12-science - Chemistry
Asked by rasmimajhi07 | 21 Nov, 2023, 10:22: PM
CBSE 12-science - Chemistry
Asked by pradeepkumar70258 | 04 Oct, 2023, 10:30: PM
CBSE 12-science - Chemistry
Asked by akash9322793205 | 12 Aug, 2021, 09:30: AM
CBSE 12-science - Chemistry
Asked by thakurranjan54 | 08 Feb, 2021, 06:32: PM
CBSE 12-science - Chemistry
Asked by sildasholly2002 | 26 Jul, 2020, 06:37: PM
CBSE 12-science - Chemistry
Asked by tejuaaygole | 11 Jul, 2020, 10:40: AM
CBSE 12-science - Chemistry
Asked by chandlerbong164 | 12 Apr, 2020, 07:22: PM
CBSE 12-science - Chemistry
Asked by mangeshkale423 | 25 Feb, 2020, 05:12: PM
CBSE 12-science - Chemistry
Asked by upasana.sobti | 16 Jan, 2019, 01:59: PM




