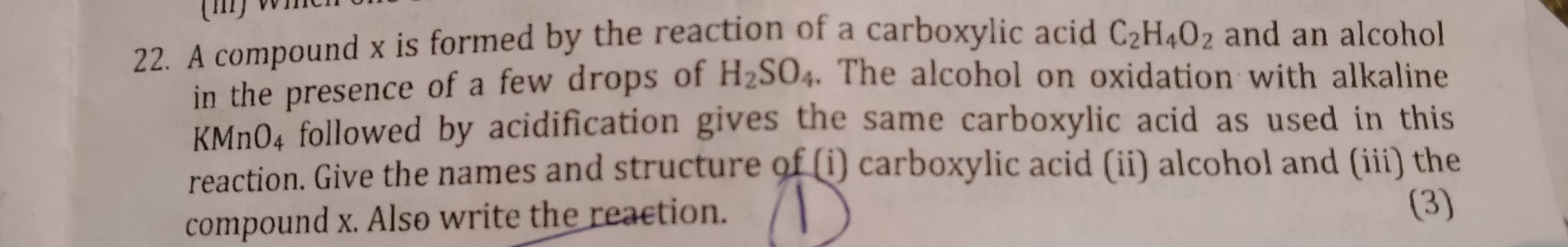CBSE Class 10 Answered
(a) (i) Ethanol is converted to ethanoic acid by the oxidation of ethanol in the presence of oxidising agent like alkaline potassium permanganate or acidified potassium dichromate.
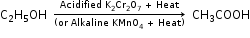
(ii) Ethanol is converted to ethene by the dehydration of ethanol in the presence of dehydrating agent conc. H2SO4.
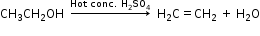
(iii) Ethanol is converted to ethyl ethanoate by the process of esterification i.e. by treating ethanol with ethanoic acid in the presence of conc. H2SO4 and warming it.
(b) Unsaturated hydrocarbons burn with a yellow flame because of incomplete combustion.
(c) Hardness of water is due to the presence of Ca2+ and Mg2+ ions. These ions react with soaps to form curdy white precipitates of calcium and magnesium salts of fatty acids.
Or
(a)The compound CH3COOH is ethanoic acid.
Its functional group is carboxylic group (-COOH).
(b) Ethanoic acid reacts with ethanol in the presence of conc. H2SO4 to form ethyl ethanoate which has a sweet smell.
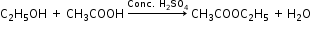
(c) Carbon dioxide is evolved when ethanoic acid reacts with solid sodium carbonate.
When the gas evolved is passed through lime water, it turns lime water milky. This shows that evolved gas is CO2.
(d) Detergents cannot be used to check whether the water is hard or not because they give a good amount of lather for both hard and soft water.