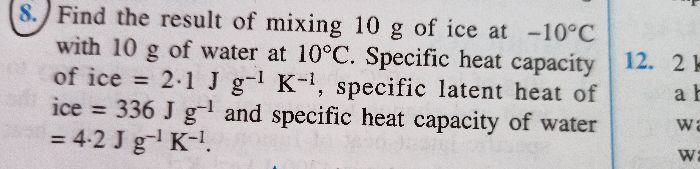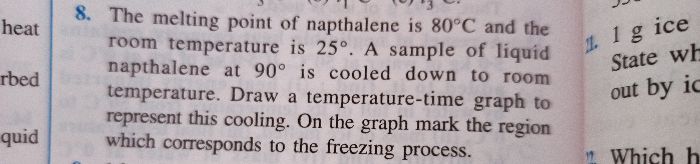ICSE Class 10 Answered
A closed calorimeter of negligible water equivalent contains 1 kg of ice at 0 degree Celsius , then 1 kg of steam at 100 degree Celsius is pumped into it. Find the ratio of mass of steam to water remaining in the calorimeter after attaining equilibrium temperature. Take the efficiency of the calorimeter as 90%. Find the amount of heat lost to the surroundings.
Asked by KSHITIJ AGRAWAL | 07 Jul, 2013, 10:58: AM

Answered by | 12 Sep, 2013, 12:53: PM
Concept Videos
ICSE 10 - Physics
Asked by imunilu786 | 29 Oct, 2023, 02:35: PM
ICSE 10 - Physics
Asked by surendrakumarclw | 20 Mar, 2021, 06:16: PM
ICSE 10 - Physics
Asked by anuradhastudent12 | 27 Feb, 2021, 08:33: PM
ICSE 10 - Physics
Asked by chitrachongdar07 | 08 Dec, 2020, 10:55: AM
ICSE 10 - Physics
Asked by charan3802 | 23 Oct, 2020, 10:29: AM
ICSE 10 - Physics
Asked by khushalkumarsk | 17 Aug, 2020, 12:37: PM
ICSE 10 - Physics
Asked by nilesh.dhote74 | 13 Jul, 2020, 12:38: PM
ICSE 10 - Physics
Asked by abhinabasarkar.abhinaba | 17 Jun, 2020, 09:24: PM
ICSE 10 - Physics
Asked by nilesh.dhote74 | 10 Jun, 2020, 03:00: PM
ICSE 10 - Physics
Asked by chaitalisen977 | 19 May, 2020, 03:32: PM