CBSE Class 11-science Answered
 1)
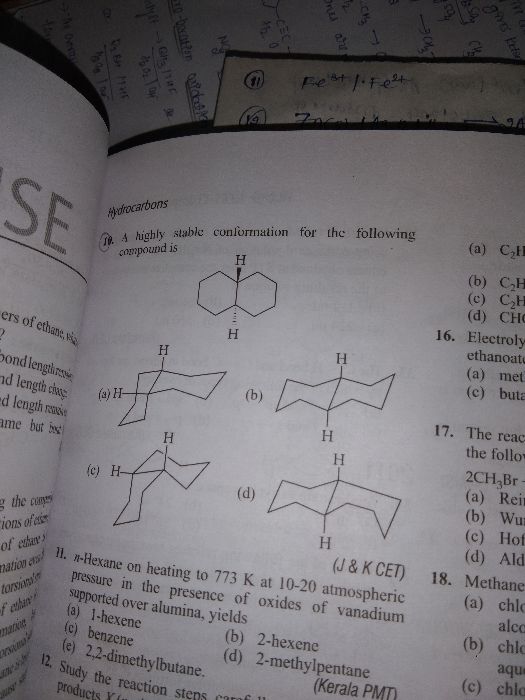
1) 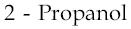 2)
2) 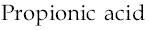 3)
3) 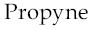 4)
4) 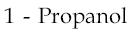
Asked by Ram Kumar | 22 Apr, 2014, 11:10: AM
Aqueous KOH results into substitution reaction.
Cl3C−CH2−CH3+3KOH(aq)→(OH)3C−CH2−CH3+3KCl
It may be noted that more than one OH group cannot be present on the same carbon atom. In such a case, the compound will be extremely unstable and will readily lose a water molecule to form more stable propionic acid.
(OH)3C−CH2−CH3?HOOC−CH2−CH3+H2O
Thus, propionic acid is the major product along with water molecule.
Hence, the corrrect option is propionic acid (option 2).
Answered by | 23 Apr, 2014, 11:39: AM
Concept Videos
CBSE 11-science - Chemistry
Asked by kirithshiv | 24 Feb, 2024, 12:12: PM
CBSE 11-science - Chemistry
Asked by shivanij4734 | 16 Dec, 2023, 08:30: PM
CBSE 11-science - Chemistry
Asked by maibamjohnny89 | 15 Jan, 2022, 09:38: PM
CBSE 11-science - Chemistry
Asked by archu312004 | 07 Feb, 2021, 10:21: PM
CBSE 11-science - Chemistry
Asked by dubeyanubhav65 | 18 Jan, 2021, 09:52: PM
CBSE 11-science - Chemistry
Asked by shahsaqlain107 | 30 Nov, 2020, 01:10: PM
CBSE 11-science - Chemistry
Asked by ghastipratiksha | 11 Jul, 2020, 08:14: PM
CBSE 11-science - Chemistry
Asked by guptaserendri | 01 Jul, 2020, 03:58: PM
CBSE 11-science - Chemistry
Asked by Manpreetsingh669933 | 13 Apr, 2020, 01:39: PM
CBSE 11-science - Chemistry
Asked by bittutiwary1234 | 23 Feb, 2020, 12:15: AM