Water, Solution, Solubility and Hydrogen
Water, Solution, Solubility and Hydrogen Synopsis
Synopsis
Water
- Water is widely distributed on the Earth, i.e. it is abundantly available on the Earth.
- More than 3/4th of the Earth’s surface or 70% of the Earth’s surface is covered with water.
- Water is found in the natural state. Oceans, frozen glaciers, fresh water on the surface of the Earth from rivers, ponds, wells etc. and water in the form of clouds is called natural water.
- Water is vital for plants, animals and human life.
- Henry Cavendish (1781) showed water to be a compound of hydrogen and oxygen.
Occurrence of Water
Water occurs in both Free State and combined state. It comprises a large part of animal and plant matter.
Free State
Combined State
Water is present in the combined state with a particular percentage.
Water Cycle
Water in its three different states keeps moving constantly from Earth to air and then back to Earth. This constant circulation of water from the Earth to the atmosphere by evaporation and back to the Earth’s surface as rain water is known as the water cycle.
Purification of Natural Water
Purification of natural water is done by two processes:
- By distillation (for chemical purpose)
- By treatment of water (for drinking purpose)
By Distillation (for chemical purpose)
Impure water can be purified by the process of distillation. Distillation is the process of converting a liquid into vapour by heating, and the subsequent condensation of the vapour back into a liquid. Water evaporates and recondenses in pure form, and it is collected in a receiver. The impurities remain in the distillation flask.
By Treatment of Water (for drinking purpose)
- Sedimentation: Settling down of particles in water by flow of water through several tanks such that bigger particles settle down at the bottom of the tank.
- Filtration: By passage of water through sand filters which consist of sand, gravel and stones.
- Chlorination: Sterilisation of water by addition of chlorine acts as a treatment against bacterial infection.
Composition of Water
When an electric current is passed through acidulated water (i.e. electrolysis), two volumes of hydrogen is formed at the cathode and one volume of oxygen is formed at the anode.
Thus, water contains Hydrogen and Oxygen in the ratio of 2:1.
Synthesis of Water
Water can be synthesised by burning hydrogen in air.
Physical Properties of Water
- Water is a colourless, odourless, tasteless, clear liquid.
- Boiling point of water is 100°C and freezing point of water is 0°C.
- Water exists in three states—Solid (ice), Liquid (water) and Gas (steam).
- Water expands on cooling.
• Water shows an unusual or anomalous behaviour when it is heated or cooled between 0°C and 4°C.
• All substances generally contract on cooling while water expands.
• When water is cooled, it first contracts like other liquids up to 4°C. On further cooling, it expands instead of contracting. This expansion takes place up to 0°C. Thus, at 0°C, water has maximum volume and minimum density. At 0°C, it becomes ice, has a density of 0.92 g/cm3 and floats on water. - On cooling, water expands in volume. Hence, the density of ice is lower than that of water. Thus, ice floats on water and fishes can survive below it.
- Water has high specific heat capacity.
A large amount of heat energy is required to raise the temperature of 1 g of water by 1°C.
A unique property of water is that it requires more heat to raise its temperature by 1°C than other specific substances. Hence, the temperature of the land near the sea is lower than the temperature of the land away from the sea.
Chemical Properties of Water
Reaction of Water with Metals
Dissolution of Oxides in Water
Tests for Water
- Water turns white anhydrous copper sulphate blue.
- Water turns blue anhydrous cobalt chloride pink.
Uses of Water
- Water is a Universal Solvent. It is used for dissolving inorganic and organic matter.
- Water is an important constituent of the human body. It is required for a large number of metabolic activities.
- Water helps control climatic conditions; these climatic conditions are influenced by the water cycle.
- Saturated solution
A solution is said to be saturated when it cannot dissolve any more of the solute at a given temperature. - Unsaturated solution
A solution which can dissolve more of the solute at a given temperature is said to be an unsaturated solution. - Supersaturated solution
If the solution contains more of the solute than which is present in its saturated solution at that particular temperature, then the solution is said to be supersaturated.
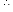 1.7 g is the solubility of NaCl in water at t°C.
1.7 g is the solubility of NaCl in water at t°C.- When pressure on the surface of water increases, solubility of a gas increases.
- When temperature of water increases, solubility of a gas decreases.
- Some salts while crystallising out from their aqueous solution, unite with a definite quantity of water which is known as water of crystallisation.
- Water of crystallisation is in loose chemical combination with salt.
- It can be driven out by heating the powdered crystals of these salts above 100°C.
Example:
When blue colour crystals of copper sulphate are heated in a test tube, they lose their water of crystallisation to form white-coloured powder of anhydrous copper sulphate.
- Caustic soda
- Caustic potash
- Magnesium chloride
- Zinc chloride
- Calcium chloride
- Ferric chloride
- Zinc nitrate
- Copper nitrate
- Concentrated sulphuric acid (H2SO4)
- Phosphorus pentoxide (P2O5)
- Quicklime (calcium oxide) (CaO)
- Silica gel (SiO2)
- Concentrated sulphuric acid (H2SO4)
- Phosphorus pentoxide (P2O5)
- Silica gel (SiO2)
- Quicklime (calcium oxide) (CaO)
- Calcium chloride (CaCl2)
Soft and Hard Water
- Water is said to be soft if it readily forms lather with soaps. Pure water or water containing sodium salts easily gives lather with soap.
- Water is said to be hard when it does not readily form lather with soap.
- Temporary Hardness:
Water which contains only hydrogen carbonates of calcium and magnesium is called temporary hard water. Its hardness can be removed by just boiling.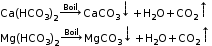
- Permanent Hardness:
Water containing sulphates and chloride of magnesium and calcium is called permanent hard water. This hardness cannot be removed by boiling.
- Soft water free from dissolved salts has a very flat taste. The presence of salts in hard water makes it tasty.
- Calcium and magnesium salts present in small amounts in hard water are essential for the growth of our bones and teeth.
- Calcium sulphate present in hard water forms insoluble lead sulphate in the form of a layer inside lead pipes and this checks lead poisoning.
- Hard water is not suitable for producing steam.
- Steam is made in boilers which are made of narrow copper tubes. As water enters these tubes, it changes to steam, while salts are incapable of changing into vapour and deposits on the inner walls of the tubes.
- This goes on and makes the bore of the tubes narrower and narrower. This results in less water flowing through the tubes and less steam is produced.
- Hard water is also unfit for washing purposes.
- If water is hard, calcium and magnesium ions of water combine with the negative ions of the soap to form a slimy precipitate of insoluble scum.
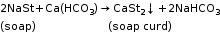
- By boiling, carbon dioxide is driven off and soluble hydrogen carbonates are converted to insoluble carbonates and can be removed by filtration or decantation.
Calcium carbonate and magnesium carbonate are precipitated leaving water soft.
Ca(HCO3)2 → CaCO3 ↓ + H2O + CO2↑
This method is not useful for a large quantity of water - By addition of washing soda:
When washing soda is added to hard water, insoluble carbonates settle and can be removed by filtration.
Ca(HCO3)2 + Na2CO3 → CaCO3 + 2NaHCO3
Water is treated with calculated quantity of soda ash to remove permanent hardness.
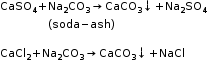
Lime is also used as magnesium carbonate is not fully precipitated.
MgSO4 + Na2CO3 + Ca(OH)2 → Mg(OH)2 + CaCO3 + Na2SO4

