Chemical Kinetics and Chemical Equilibrium
Chemical Kinetics and Chemical Equilibrium Synopsis
Synopsis
Rate of Chemical Reaction
Rate of Chemical Reaction:
- Alternately the rate of reaction can also be expressed as,
- Consider a hypothetical reaction, assuming that the volume of the system remains constant.
R → P
One mole of the reactant R produces one mole of the product P.
- If [R]1 and [P]1 are the concentrations of R and P respectively at time t1 and [R]2 and [P]2 are their concentrations at time t2 then,
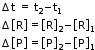
The square brackets in the above expressions are used to express molar concentration.
- Δ[R] is a negative quantity because concentration of reactants is decreasing.
- Equations (1) and (2) given above represent the average rate of a reaction, rav.
This average rate depends upon the change in concentration of reactants or products and the time taken for that change to occur.
Units of rate of a reaction:
- From equations (1) and (2), it is clear that units of rate are concentration time–1.
- For example, if concentration is in mol L-1 and time is in seconds then the units will be mol L-1s-1.
- In gaseous reactions, the concentration of gases is expressed in terms of their partial pressures; hence the units of the rate equation will be atm s-1.
Instantaneous Rate of Reaction:
- Consider a hydrolysis of butyl chloride (C4H9Cl).
C4H9Cl + H2O → C4H9OH + HCl - We have provided the concentrations over different intervals of time below.
- We can determine the difference in concentration over different intervals of time and thus determine the average rate by dividing Δ[R] by Δt.
- It can be seen from experimental data that the average rate falls from 1.90 × 10-4 mol L-1s-1 to 0.4 × 10-4 mol L-1s-1.
- However, average rate cannot be used to predict the rate of a reaction at a particular instant as it would be constant for the time interval for which it is calculated.
- Hence, to express the rate at a particular moment of time we determine the instantaneous rate.
- It is obtained when we consider the average rate at the smallest time interval say dt, when Δt approaches zero.
Therefore, for an infinitesimally small dt, instantaneous rate is given by,
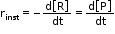
- By drawing the tangent at time t on the either of the curves for concentration of R vs time t or concentration of P vs time t and calculating the slope of the curve, we can determine the instantaneous rate of reaction.
- Hence, here in this example rinst at 600s is calculated by plotting graph of concentration of butyl chloride as against time t.
- A tangent is drawn on the curve at a point t = 600s.
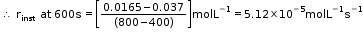
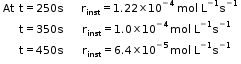
- Now consider a reaction,
Hg(l) + Cl2(g) → HgCl2(s)
Here, the stoichiometric coefficients of the reactants and products are same; hence rate of the reaction is given as,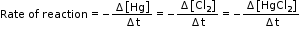
Therefore, we can say that from above equation that the rate of disappearance of any of the reactants is same as the rate of appearance of the products.
- Consider another reaction,
2 HI(g) → H2(g) + I2(g)
In this reaction, two moles of HI decompose to produce one mole each of H2 and I2 i.e. the stoichiometric coefficients of reactants or products are not equal to one; hence we need to divide the rate of disappearance of any of the reactants or the rate of appearance of products by their respective stoichiometric coefficients.
Since rate of consumption of HI is twice the rate of formation of H2 or I2, to make them equal, the term Δ[HI] is divided by 2.
The rate of this reaction is given by,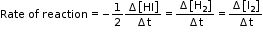
- For a gaseous reaction at constant temperature, concentration is directly proportional to the partial pressure of a species and hence, rate can be expressed as rate of change in partial pressure of the reactant or the product.
Factors affecting the rate of reaction:

