Chemical Bonding
Chemical Bonding Synopsis
Synopsis
• Chemical Bond
A chemical bond is defined as the force of attraction between any two atoms in a molecule to maintain stability.
• Noble Gases
- Have stable electronic configuration, i.e. their outermost shell is complete.
- They have 2 electrons in the outermost shell or 8 electrons in the outermost shell.
- They do not lose, gain or share electrons and are inert or unreactive.
• Atoms of Elements – Other than Noble Gases
- Have unstable electronic configuration, i.e. their outermost shell is incomplete.
- They can lose, gain or share electrons and are chemically reactive.
• Reasons for Chemical Bonding
- The driving force for atoms to combine is related to the tendency of each atom to attain stable electronic configuration of the nearest inert noble gas.
- For an atom to achieve stable electronic configuration, it must have
- Two electrons in the outermost shell (nearest noble gas He) – Duplet rule
- Eight electrons in the outermost shell (all noble gases other than He) – Octet rule
• Methods for Achieving Chemical Bonding
There are three methods in which atoms can achieve a stable configuration.
- Transfer of one or more electrons from one atom to the other to form an electrovalent bond.
- Sharing of one, two or three pairs of electrons between two atoms to form a covalent bond.
- When the shared electron pairs are contributed by only one of the combining atoms, the bond formed is known as a coordinate bond.
Electrovalent (or Ionic) Bond
• Types of Elements
- Metallic elements have 1, 2 or 3 electrons in their valence shell. They lose 1, 2 or 3 electrons and become positively charged ions [cations].
- Non-metallic elements have 4, 5, 6 or 7 electrons in their valence shell. They gain (4), 3, 2 or 1 electrons and become negatively charged ions [anions].
• Ionic Bond
The chemical bond formed between two atoms by transfer of one or more electrons from the atom of a metallic electropositive element to an atom of a non-metallic electronegative element.
• Ionic Compound
The chemical compound formed as a result of transfer of one or more electrons from the atom of a metallic electropositive element to an atom of a non-metallic electronegative element.
• Electrovalency
The number of electrons donated or accepted by the valence shell of an atom of an element so as to achieve stable electronic configuration is called electrovalency.
• Conditions for the Formation of an Ionic Bond
- Ionisation Potential (IP)
Lower the value of IP of a metallic atom, greater the ease of formation of the cation. - Electron affinity
Higher the value of EA of a non-metallic atom, greater the ease of formation of the anion. - Electronegativity
Larger the electronegativity difference between combining atoms, greater the ease of electron transfer.
• Stability of Ionic Compounds
- Ionic compounds are formed by ions, but there also exists a repulsive force between ions for like charges.
- Since the electrostatic force of attraction between opposite charges is much higher, it makes ionic compounds stable.
Examples: NaCl, MgCl2, CaO, KBr, CaCl2
• Formation of Electrovalent Compounds
A metallic atom loses electrons to attain a stable electronic configuration and becomes a cation.

A non-metallic atom gains electrons to attain a stable electronic configuration and becomes an anion.

Cations and anions are oppositely charged particles which attract one another to form an electrovalent bond leading to the formation of an electrovalent compound.
1. Formation of Sodium Chloride:
The electronic configuration of sodium atom is 2, 8, 1. Since its valency is 1, it loses one electron to attain the stable electronic configuration of neon and becomes a positively charged sodium ion (Na+) having a net charge +1.
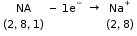
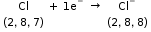
The electronic configuration of chlorine atom is 2, 8, 7. As its valency is 1, it will accept one electron to complete its octet and attain stability.
So, it will accept the one electron lost by the sodium atom to become a chloride ion (Cl−) having −1 net charge.
So, cation Na+ and anion Cl− are attracted towards each other due to electrical charge and form an ionic compound, sodium chloride.
Na + Cl → Na+ Cl− → NaCl
• Atomic or Orbit Structural Diagram
Electron Dot Structural Diagram
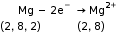
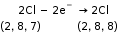
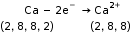
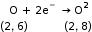
· Atomic or Orbit Structural Diagram:
- Single covalent bond
A single bond is formed by the sharing of one pair of electrons between the atoms, each atom contributing one electron. It was denoted by putting a short line (–).
Examples: Hydrogen (H2), chlorine (Cl2) - Double covalent bond
A double bond is formed by the sharing of two pairs of electrons between two atoms. It is represented by putting two short lines (=) between the two atoms.
Examples: Oxygen molecule (O=O), carbon dioxide (O=C=O) - Triple covalent bond
A triple covalent bond is a combination of three single bonds, represented by putting three short lines.
Examples: Nitrogen molecule(N≡N), ethyne(HC≡CH)
- Both atoms should have high electronegativity, electron affinity and ionisation energy.
- The electronegative difference between the two combining atoms should be negligible.
- Both atoms should have four or more electrons in their outermost shell.
- Hydrogen molecule (Non-polar molecule):
A hydrogen atom has only one electron in its K shell. It needs one more electron to complete its duplet or to attain the stable electronic configuration of the nearest rare gas helium. To meet this need, the hydrogen atom shares its electron with another hydrogen atom to form a hydrogen molecule.
Formation of hydrogen molecule: - Chlorine molecule:
- Nitrogen molecule:
- Formation of Water – Polar Covalent Compound
- Formation of an ammonia molecule:
- Carbon tetrachloride molecule (Non-polar molecule):
One atom of carbon shares four electron pairs, one with each of the four atoms of hydrogen.
Coordinate Bond
The bond formed between two atoms by sharing a pair of electrons provided entirely by one of the combining atoms but shared by both is called a coordinate bond or dative bond.
Examples: Ammonium ion (NH4+), hydronium ion (H3O+)
- One of the two atoms must have at least one lone pair of electrons. Examples: Ammonia (NH3), water (H2O)
- Another atom should be short of at least one lone pair of electrons. Example: Hydrogen ion (H+)
- Oxidation
When an atom or ion loses an electron or electrons, oxidation takes place.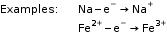
- Reduction
When an atom or ion gains an electron or electrons, reduction takes place.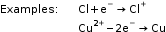
- Oxidising and Reducing Agents
The atom or ion which gains an electron or electrons is an oxidising agent.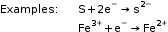
The atom or ion which loses an electron or electrons is a reducing agent.
Example: Al – 3e– → Al3+
- Redox Reaction
A chemical reaction in which loss of electrons and the gain of electrons take place simultaneously is called a redox reaction.
Example:

