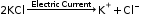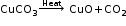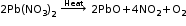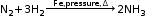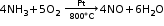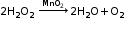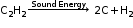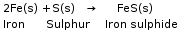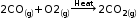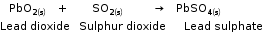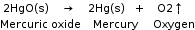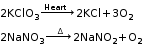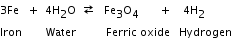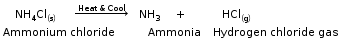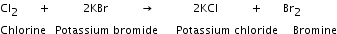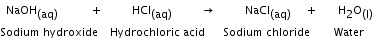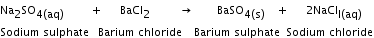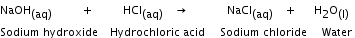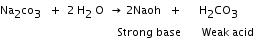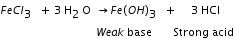Nature of Matter
Nature of Matter Synopsis
Synopsis
Kinetic theory of Matter
- Molecules of matter are in a continuous state of motion. Hence, they possess kinetic energy.
- The kinetic energy of molecules increases with an increase in temperature and decreases with a decrease in temperature.
- The molecules of matter always attract each other and the force of attraction between them is known as force of cohesion which binds the molecules of a substance together.
- This force is maximum between the molecules of solids, lesser between the molecules of liquids and least between the molecules of gases.
- The force of attraction between the different types of molecules is called the force of adhesion.
- The force of attraction between the molecules (either cohesive or adhesive) is called the intermolecular force of attraction.
- The space between any two consecutive molecules is called the intermolecular space. If the intermolecular force of attraction increases, the intermolecular space decreases, and vice versa.
• States of Matter
|
Solid State |
Liquid State |
Gaseous State |
|
|
|
|
|
The space between the particles is very less.
|
The space between the particles is slightly more as compared to the solids, but still very less as compared to the gases. The particles of liquid can slip and slide over each other. |
The particles are much farther apart from one another as compared to the solids and liquids. They have a very disorderly arrangement of particles compared to the solids and liquids. |
|
The force of attraction between the particles is strong. Thus, particles in a solid are closely packed.
|
The force of attraction between the particles is strong enough to hold the particles together but not strong enough to hold the particles in a fixed position. |
The force of attraction between the particles is negligible, hence particles of a gas move freely in all the directions. Gases thus can mix or diffuse into other gases. |
|
The kinetic energy of the particles is very less and so solids have an orderly arrangement of the particles. Therefore, solids have a fixed shape and a fixed volume. |
The kinetic energy of the particles is more than that of the solids. Thus, liquids have a disorderly arrangement of particles compared to solids. |
The particles of a gas have maximum kinetic energy. They move with high speed in all directions and can exert pressure on the walls of its container. |
|
Solids maintain their shape even when they are subjected to external force i.e. they are rigid. |
Liquids do not have a fixed shape but have a fixed volume. Liquids take up the shape of the container in which they are poured. |
Gases neither have a definite shape nor a definite volume. They fill up the container completely. |
|
Solids cannot be compressed. |
Liquids cannot be compressed much. The compressibility of liquids is almost negligible. |
Gases can be compressed easily. Example: the LPG cylinders used at home and the CNG cylinders used in vehicles. |
|
Some solids may change their shape when an external force is applied but when that force is removed they can regain their original shape. This shows that some solids are elastic. |
Liquids show a property called viscosity. More viscous liquids flow more slowly, while less viscous liquids flow easily.
|
__ |
Change of State of Matter (Phase transition)
- The phenomenon of change from one state of matter to another, and then back to the original state is called the inter-conversion of states of matter.
- Matter can be transformed from one state to another state by changing the temperature or pressure. This transformation is called a change in state.
Effect of Change of Temperature
- On increasing the temperature of solids, the kinetic energy of the particles increase.
- Due to the increase in kinetic energy, the particles start vibrating with a greater speed.
- The energy supplied by heat overcomes the forces of attraction between the particles.
The particles leave their fixed position and start moving freely. - A stage is reached when the solid melts and is converted to a liquid.
Melting point: (Solid → Liquid)
The temperature at which a solid melts to become a liquid at the atmospheric pressure is called its melting point.
Melting point is the characteristic property of a substance. For example, melting point of ice is 0°C (273 K).
Latent heat: The hidden heat which breaks the force of attraction between the molecules is known as the latent heat. Since, the heat energy is hidden in the bulk of the matter, it is called latent heat.
Latent heat of fusion: The heat energy required to convert 1 kilogram of solid into liquid at the atmospheric pressure, at its melting point is known as the latent heat of fusion.
- When we supply heat energy to water, the particles start moving even faster.
- At a certain temperature, a point is reached when the particles have enough energy to break free from the forces of attraction of each other.
- At this temperature, the liquid starts changing into a gas.
Boiling Point: (Liquid → Gas)
- The temperature at which a liquid starts boiling at the atmospheric pressure is called its boiling point.
- Boiling is a bulk phenomenon.
- Particles from the bulk of the liquid gain energy to change into vapour state.
- For example, boiling point of water is 100°C. (Or 100°C = 273 + 100 = 373 K)
Latent heat of vaporisation: The heat energy required to convert 1 kilogram of liquid into gas at the atmospheric pressure at its boiling point is known as the latent heat of vaporisation.
- The process, in which a gas, on cooling, turns into a liquid at a specific temperature is called condensation or liquefaction.
- During condensation, the particles of the gas lose kinetic energy and come closer to each other until they start being attracted to each other and form a liquid.
- Formation of clouds is due to the condensation of water vapour from the Earth’s surface.
- The heat removed from the surface through evaporation is released in the atmosphere by the formation of clouds. This process cools the Earth’s climate.
- The change of state of a substance directly from a solid to gas, without changing into a liquid state (or vice versa) is called sublimation.
- The common substances which undergo sublimation are camphor, naphthalene, ammonium chloride, solid carbon dioxide and iodine.
- The gases can be liquefied by applying pressure and reducing the temperature.
- When a high pressure is applied to a gas, it gets compressed and if the temperature is lowered, the gas is liquefied. Let us understand this.
- In a gaseous substance there is a lot of space between the particles. If pressure is applied, the particles of the gas come closer and the gas is compressed.
- When a gas is compressed, heat is produced due to compression.
- So, while applying pressure it is necessary to decrease the temperature, in order to take away the heat produced during compression.
- An element is made up of only one kind of atoms.
- An element is pure and homogeneous substance.
- An element has fixed melting and boiling points.
- An atom is the smallest particle of an element which takes part in a chemical reaction.
- An element may chemically react with another elements or compounds.
- An element can occur in solid, liquid or gaseous state.
- The molecules are made up of one or more atoms of the same or different elements.
- Components in a compound are present in a definite proportion.
- It has a homogeneous composition.
- Particles in a compound are of one kind.
- A compound is made up of one or more atoms of the same or different elements.
- In a compound the elements are present in a fixed ratio by mass.
- A compound can be divided into simpler substances by a chemical process.
- The physical and chemical properties of a compound are completely different from those of its constituents.
Separating the Components of a Mixture
- To obtain the coloured component of a dye from blue/black ink
- Separation of Cream from Milk
- To separate a mixture of two Immiscible liquids
- To separate a mixture of Salt and Ammonium chloride
- Separation of Components of Dye
- To separate a mixture of two miscible liquids
- To separate a mixture of two miscible liquids having the temperature difference less than 25°C.
- To obtain different gases from air
- To Obtain Pure Copper sulphate Crystals From An Impure Samplexc
- To Separate The Mixture of Iron Filings and Sulphur Powder
|
Close physical contact (Mixing) |
A chemical reaction occurs when two substances are mixed in their solid state. |
|
Solution |
A chemical reaction occurs when two substances are mixed in the solution form. |
|
Heat |
Some chemical reactions occur only on heating.
|
|
Light |
Reactions which occur by the action of light are called photochemical reactions or photolysis. Molecules of the reactants absorb light energy, get activated and then react rapidly. |
|
Electricity |
Chemical reactions such as decomposition of compounds occur only when electricity is passed through the substance. |
|
Pressure |
Some reactions occur only when substances are subjected to high pressure. |
|
Catalyst |
Some chemical reactions need a catalyst to accelerate or decelerate their rates of reaction. Catalysts themselves do not take part in the reaction. A catalyst such as Pt or MnO2 initiates a change in the rate of the reaction without undergoing any change in its chemical composition. Positive catalyst: A positive catalyst accelerates a reaction.
|
|
Sound |
Some chemical reactions proceed only by absorption of sound energy. Sound energy speeds up the reacting molecules, atoms or ions causing a reaction to occur. |
|
1. Evolution of gas |
One of the products in a chemical reaction is a gas. |
|
2. Change of colour |
Some chemical reactions are characterised by a change in the colour of the reactants. |
|
3. Formation of precipitate |
Some chemical reactions are characterised by the formation of precipitate. The precipitate is an insoluble solid substance. |
|
4. Change of state |
In some reactions, a change of a state is observed. The reaction starts with solid or liquid reactants and ends up with gaseous products and vice versa. |
|
1. Direct combination or synthesis |
A chemical reaction in which two or more substances combine to form a single product. 1) Combination of two elements: When iron and sulphur are heated together, they combine to form a single product, iron sulphide. 2) Combination of an element and a compound: Carbon dioxide (a compound) burns in the presence of oxygen (an element) to form carbon dioxide. 3) Combination of two or more compounds: When lead dioxide is reacted with sulphur dioxide, both these compounds combine to form lead sulphate. |
|
2. Decomposition reaction |
A chemical reaction in which a single compound splits into two or more simple substances. When mercuric oxide is heated in a crucible, the orange–red powder begins to darken and a silver mirror begins to deposit on the cooler parts of the crucible. This is mercury. If we hold a glowing splint near the crucible, it can relight. This shows that the gas evolved during the reaction is oxygen.
Decmposition occurs by application of heat or light or by the passage of electric current. |
|
|
Electrolysis of acidulated water: On passing electric current through acidulated water, water produces two volumes of hydrogen gas and one volume of oxygen gas.
|
|
|
Thermal decomposition: A decomposition reaction brought about by heat. During thermal decomposition, a chemical compound breaks into simpler compounds. The simpler compounds do not reunite to form the original compound on cooling.
|
| 3. Reversible reaction
|
A reaction in which the direction of a chemical change can be easily reversed by changing the conditions under which the reaction is taking place. The reaction between iron and water to produce ferric oxide and hydrogen gas is a reversible reaction. This is indicated by the use of a two-way arrow.
|
|
|
Thermal dissociation A reaction in which a substance dissociates into two or more simpler substances on the application of heat is called a thermal dissociation reaction. It is a reversible reaction. Ammonium chloride dissociates in the presence of heat to form ammonia and hydrogen chloride gas. This is the forward reaction. Ammonia and hydrogen chloride combine at a low temperature to form ammonium chloride. This is the reverse reaction.
|
|
4. Displacement reaction |
A reaction in which the more reactive element displaces the less reactive element from its compound. Zinc displaces copper in copper sulphate to form zinc sulphate.
Chlorine being more electronegative than potassium displaces it from potassium bromide to form potassium chloride and bromine gas. |
|
5. Double displacement |
A reaction in which ions of the reactants exchange places to form two new compounds. In a double displacement reaction, the two reactants taking part are generally water soluble, and one of the products is soluble and the other being insoluble separates out as a solid. Sodium hydroxide reacts with hydrochloric acid to form sodium chloride and water.
|
|
6. Double decomposition |
A type of chemical change in which two compounds in a solution react to form two new compounds by the mutual exchange of radicals. Usually, a solid is formed as a result of the reaction. These reactions may also be accompanied with the evolution of gas. FeS(s) + H2SO4(aq) → FeSO4 + H2S↑ These reactions are of two types—precipitation reaction and neutralisation reaction. |
|
|
Precipitation reaction The insoluble solid formed during a double displacement reaction is called a precipitate. A reaction in which a precipitate is formed as one of the products is called a precipitation reaction. Sodium sulphate reacts with barium chloride to form barium sulphate and sodium chloride solution.
|
|
|
Neutralisation reaction The reaction between an acid and a base to form a salt and water is called a neutralisation reaction.
Uses of neutralisation reaction in everyday life: a) Venom of honey bee contains formic acid. When someone is stung by a bee, formic acid enters the skin and produces pain which can be relieved by rubbing the spot with slaked lime or baking soda, both of which are bases. b) Acidity is caused by excess secretion of HCl by stomach glands. It can be relieved by taking milk of magnesia or sodium hydrogen carbonate, both of which are bases. On the other hand, deficiency of HCl is covered up by taking any suitable acid in the dilute form. c) Acid which is spilled on to our clothes or body can be neutralised with ammonia solution. d) If soil becomes somewhat acidic and thus unfavourable for growing certain crops, then slaked lime is added to neutralise the excess acid. |
|
|
Hydrolysis It is the process in which a salt and water react to form an acidic or basic solution. 1) Hydrolysis of a salt formed by the reaction of a strong base and a weak acid forms a basic solution which turns red litmus blue. Example: 2) Hydrolysis of a salt formed by the reaction of a strong acid and a weak base forms an acidic solution which turns blue litmus red. Example:
|
Chemical energy
Each substance has a fixed amount of stored energy which is in the form of potential energy. This energy is called its chemical energy.
Effervescence
The formation of gas bubbles in a liquid during a reaction is called effervescence.
Exothermic change
A chemical change which takes place with the release of heat energy is called an exothermic change.
- Carbon burns in oxygen to form carbon dioxide, and heat energy is produced.
C + O2 → CO2 + Heat - When water is added to quick lime (calcium oxide), slaked lime (calcium hydroxide) is produced with a lot of heat energy.
CaO + H2O → Ca(OH)2 + Heat - Formation of water
2H2 + O2 → 2H2O + Heat - Formation of ammonia
N2 + 3H2 → 2NH3 + Heat
Endothermic change
A chemical change which takes place with the absorption of heat energy is called an endothermic change.
- Formation of carbon disulphide
C + 2S + Heat → CS2 - When nitrogen and oxygen are heated together to a particular temperature of about 3000°C, nitric oxide gas is formed.
N2 + O2 + Heat → 2NO - Calcium carbonate decomposes into carbon dioxide and calcium oxide when heated to a temperature of about 1000°C.
CaCO3 + Heat → CaO + CO2
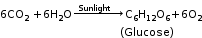
A chemical reaction which proceeds with the absorption of light energy.
Example: Photosynthesis in plants

Electrochemical reaction
A chemical reaction which proceeds with the absorption of electric energy.
Example: On passing electric current, fused potassium chloride breaks into its charged particles (ions).