Metals and Non-Metals
Metals and Non-Metals Synopsis
Synopsis
Physical Properties of Metals
Difference in Physical Properties of Metals and Non-Metals:
Chemical Properties of Metals
Reaction of Metals with Oxygen
Almost all metals react with oxygen to form metal oxides.
- Sodium and potassium are the most reactive and react with oxygen present in the air at room temperature to form the oxides.
- Magnesium does not react with oxygen at room temperature, but on heating, it burns in the air with intense light and heat to form magnesium oxide.
Reaction of Metals with Water
Metals react with water to produce metal oxides with the release of hydrogen gas. But all metals do not react with water.
- Metals such as sodium and potassium react vigorously with cold water to lead to evolution of hydrogen, which immediately catches fire producing a large quantity of heat.
- Metals such as aluminium, zinc and iron do not react with cold or hot water, but they react with steam to form metal oxides and hydrogen.
Reactions of Metals with Acids
Metals react with acids to form salt and hydrogen gas.
- i.Metals react with dilute hydrochloric acid to give metal chloride and hydrogen gas.
Mg + 2HCl → MgCl2 + H2 - Metals react with sulphuric acid to form metal sulphate and hydrogen gas.
Fe + H2SO4 → FeSO4 + H2 - Metals react with nitric acid, but hydrogen gas is not evolved since nitric acid is a strong oxidising agent. So, it oxidises the hydrogen to water and itself gets reduced to a nitrogen oxide.
But magnesium and manganese react with dilute nitric acid to evolve hydrogen gas.
Mg + 2HNO3 → Mg (NO3)2 + H2
Mn + 6HNO3 → Mn (NO3)2 + H2
Reactivity Series
The arrangement of metals in the order of decreasing reactivities is called the reactivity series of metals.
Reactions of Metals with Solutions of Other Metal Salts
A more reactive metal displaces a less reactive metal from its salt solution.
For example:
When an iron nail is placed in a copper sulphate solution, the blue colour of CuSO4 fades away slowly and a reddish brown copper metal is formed.
CuSO4(aq) + Fe(s) → FeSO4(aq) + Cu(s)
Reaction of Metals with Chlorine
Metals react with chlorine to form metal chlorides.
For example:
- Sodium readily reacts with chlorine to form ionic chloride called sodium chloride.
2Na(s) + Cl2(g) → 2NaCl(s) - Calcium reacts vigorously with chlorine to form calcium chloride.
Ca(s) + Cl2(g) → 2CaCl2(s)
Properties of Ionic Compounds
- Ionic compounds are hard solids, due to the strong force of attraction between the positive and negative ions.
- They are generally brittle and break into pieces when pressure is applied.
- Ionic compounds have high melting and boiling points, since a large amount of energy is required to break the strong intermolecular attractions.
- They are soluble in water, but insoluble in solvents such as kerosene, petrol, etc.
- They do not conduct electricity in a solid state, because electrostatic forces of attraction between ions in the solid state are very strong but conduct electricity in the fused (or in the aqueous state) because these forces weaken in the fused (or in solution) state so that their ions become mobile.
Metallurgy
Minerals: The naturally occurring compounds of metals, along with other impurities are known as minerals.
Ores: The minerals from which metals are extracted profitably and conveniently are called ores.
Gangue: Earthly impurities including silica, mud, etc. associated with the ore are called gangue.
Metallurgy: The process used for the extraction of metals in their pure form from their ores is referred to as metallurgy.
Extraction of Metals
- The reactivity of elements differs for different metals.
- Three major steps involved in the extraction of metals from their ores are:
Conversion of Concentrated Ore into Metal
- The extraction of a metal from its concentrated ore is essentially a process of reduction of the metal compound present in the ore.
- The method of reduction to be used depends on the reactivity of the metal to be extracted.
• Extraction of Less Reactive Metals
Metals at the bottom of the reactivity series are not very reactive and the oxides of these metals can be reduced by heating the ore itself.
Extraction of Mercury
Cinnabar, an ore of mercury is first heated in the air and is converted into mercuric oxide.
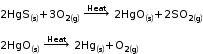
• Extraction of Moderately Reactive Metals
- The moderately reactive metals in the middle of the reactivity series are extracted by the reduction of their oxides with carbon, aluminium, sodium or calcium.
- It is easier to obtain metals from their oxides (by reduction) than from carbonates or sulphides. So, before reduction can be done, the ore is converted into a metal oxide.
- The concentrated ores can be converted into metal oxides by the process of calcination or roasting.
Calcination is the process in which a carbonate ore is heated strongly in the absence of air to convert it into a metal oxide.
For example:

Roasting is the process in which a sulphide ore is strongly heated in the presence of air to convert it into a metal oxide.

The metal oxides are converted to free metal by using reducing agents such as carbon, aluminium, sodium or calcium.
For example:
- The metal zinc is extracted by the reduction of zinc oxide with carbon. Thus, when zinc oxide is heated with carbon, zinc is produced.
- Aluminium reduces iron oxide to produce the metal iron with the evolution of heat. Due to this heat, the iron is produced in the molten state.
Fe2O3(s) + 2Al(s) → 2Fe(l) + Al2O3(s) + Heat
The reaction of iron (III) oxide with aluminium is used to join railway tracks or cracked machine parts. This reaction is known as the thermite reaction.
• Extraction of Highly Reactive Metals
Metals high up in the reactivity series are very reactive.
These metals have a strong affinity for oxygen. So, oxides of sodium, magnesium, calcium and aluminium cannot be reduced by carbon.
These metals are obtained by electrolytic reduction.
Sodium, magnesium and calcium are obtained by the electrolysis of their molten chlorides.
For example:
Sodium metal is extracted by the electrolytic reduction of molten sodium chloride.
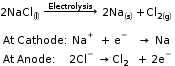
Refining of Metals
- The most widely used method for refining impure metals is electrolytic refining.
- Electrolytic refining means refining by electrolysis. Metals such as copper, zinc, tin, lead, chromium, nickel, silver and gold are refined electrolytically.
Corrosion
- When the surface of a metal is attacked by air, moisture or any other substance around it, the metal is said to corrode and the phenomenon is known as corrosion.
- Conditions necessary for rusting of iron
- Presence of air (or oxygen)
- Presence of water (or moisture)
Prevention of Corrosion
- Galvanising: It is the process of giving coating a thin layer of zinc on iron or steel to protect them from corrosion. Example: shiny nails, pins. etc.
- Tinning: It is a process of coating tin over other metals.
- Electroplating: In this method, a metal is coated with another metal using electrolysis. Example: silver plated spoons, gold plated jewellery etc.
- Alloying: An alloy is a homogeneous mixture of two or more metals or a metal and a non-metal in a definite proportion. The resultant metals, called alloys do not corrode easily.
For example: Brass (copper and zinc), Bronze (copper and tin) and Stainless steel (iron, nickel, chromium and carbon).
Download complete content for FREE 

