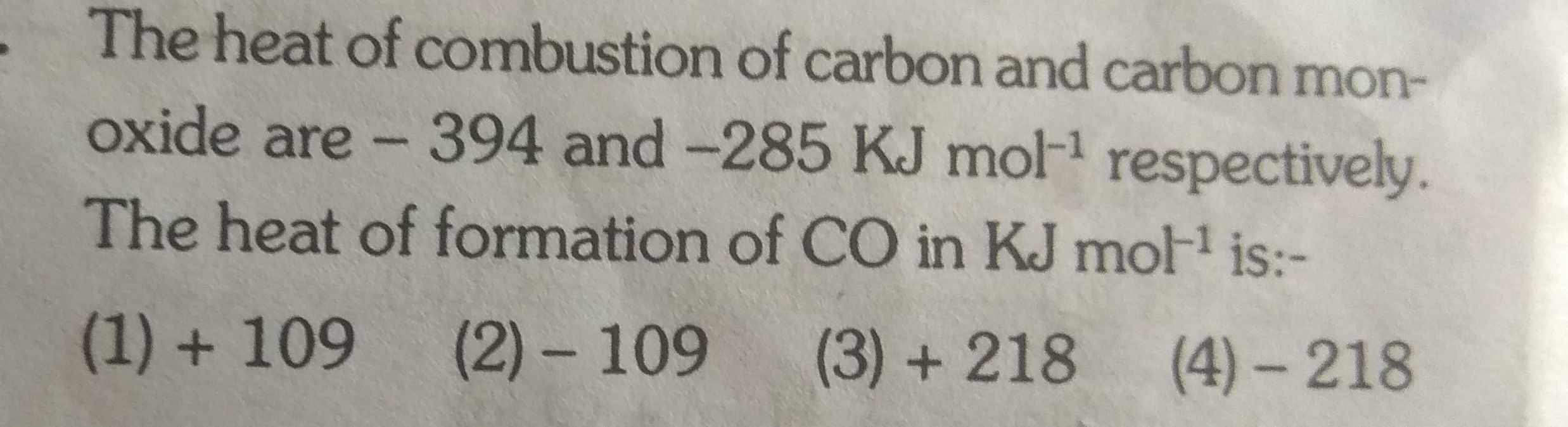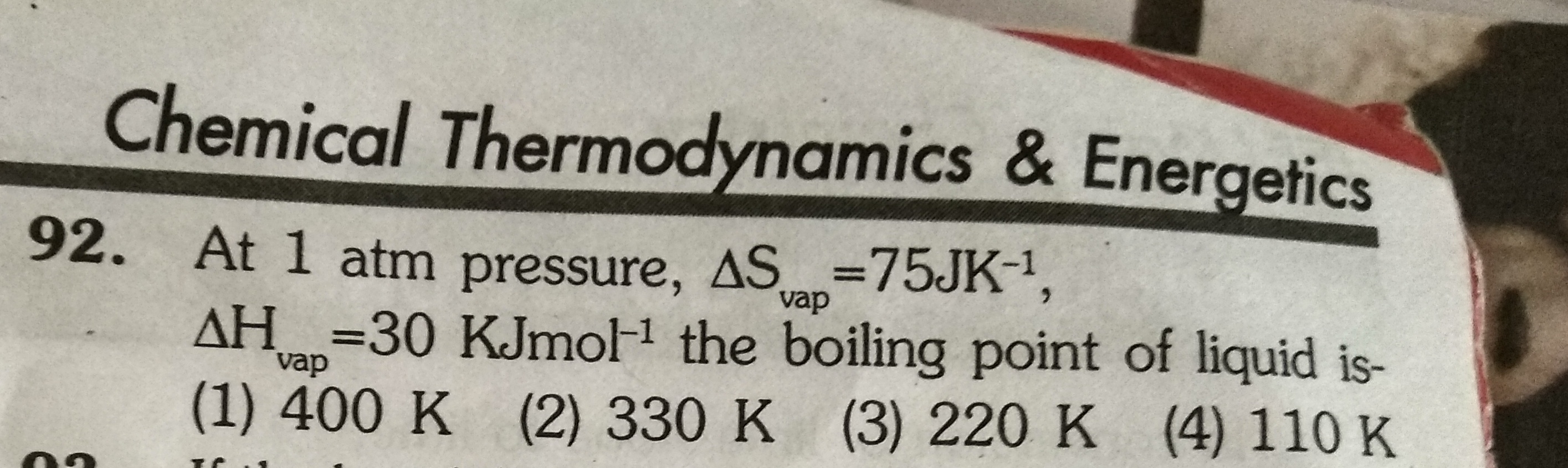JEE Class main Answered
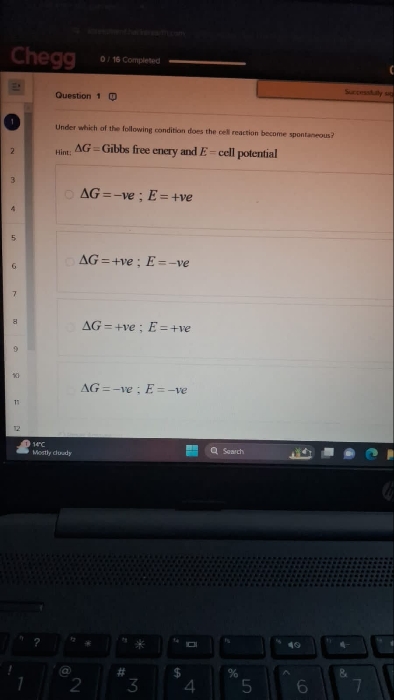
The standard change in Gibbs energy for the reaction, at 25°C is:
a)
100 kJ
b)
-90 kJ
c)
90 kJ
d)
-100 kJ
Asked by Ranjeetgupta26068 | 19 May, 2019, 10:14: PM
Formula used to find ΔGº
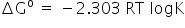
K for H2O = 10-14/55.6
Solution:

Answered by Ramandeep | 22 May, 2019, 06:28: PM
JEE main - Chemistry
Asked by ashutosharnold1998 | 03 Nov, 2019, 08:22: PM
JEE main - Chemistry
Asked by ashutosharnold1998 | 31 Oct, 2019, 07:16: PM
JEE main - Chemistry
Asked by ashutosharnold1998 | 10 Aug, 2019, 12:10: AM
JEE main - Chemistry
Asked by Ranjeetgupta26068 | 19 May, 2019, 10:14: PM
JEE main - Chemistry
Asked by g_archanasharma | 20 Feb, 2019, 05:23: PM
JEE main - Chemistry
Asked by g_archanasharma | 08 Feb, 2019, 05:48: PM