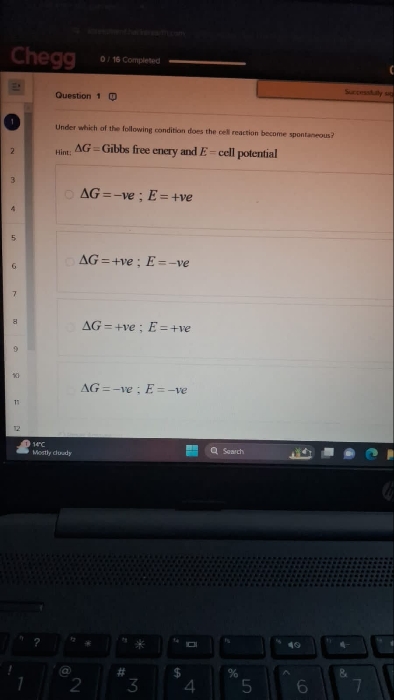
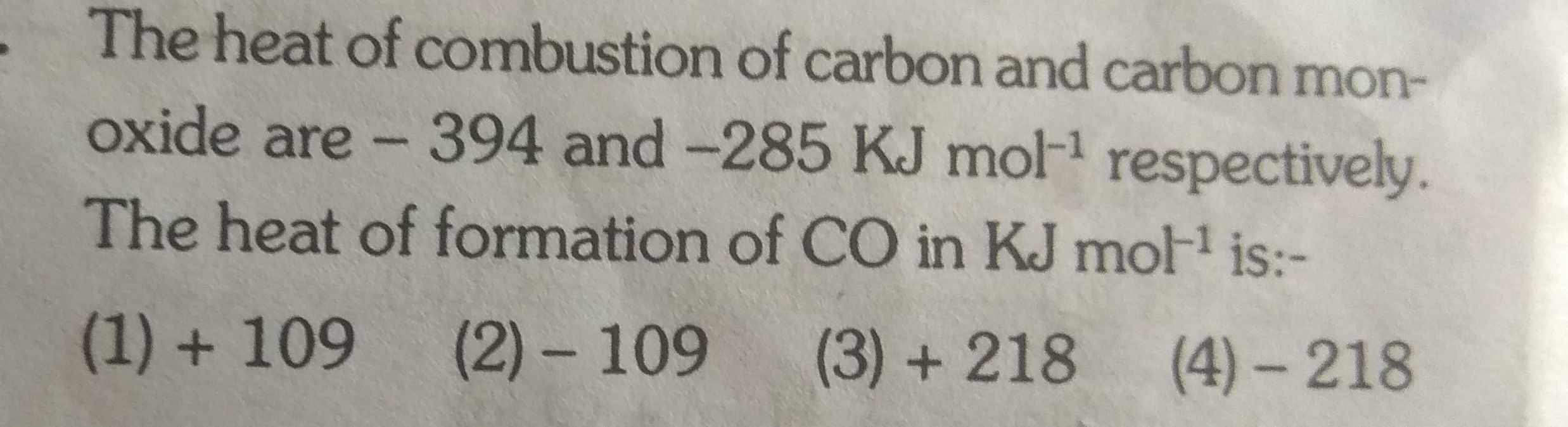JEE Class main Answered
Detailed solution please.
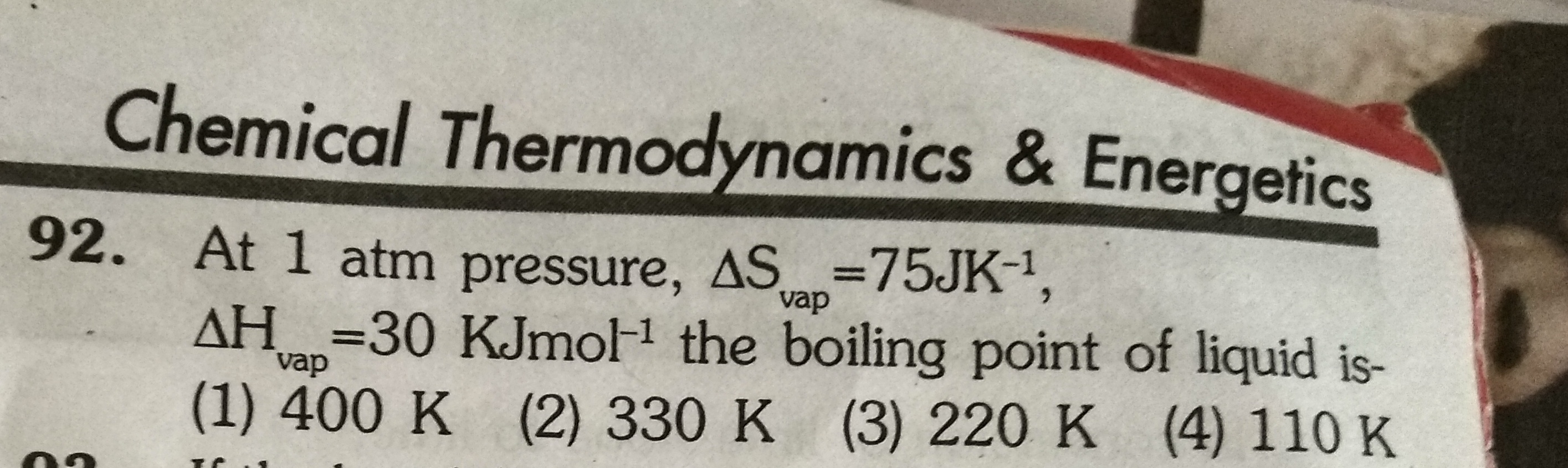
Asked by g_archanasharma | 08 Feb, 2019, 17:48: PM
Given:
ΔSvap = 75 J/K
ΔH = 30 KJ/mol
= 30000 J/mol
At boiling point,
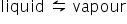 is in equilibrium,
is in equilibrium,ΔG = 0
We have,
ΔG = ΔH -TΔS
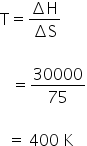
The boiling point of the liquid is 400 K.
Answered by Varsha | 08 Feb, 2019, 18:51: PM
JEE main - Chemistry
Asked by ashutosharnold1998 | 03 Nov, 2019, 20:22: PM
JEE main - Chemistry
Asked by ashutosharnold1998 | 31 Oct, 2019, 19:16: PM
JEE main - Chemistry
Asked by ashutosharnold1998 | 10 Aug, 2019, 00:10: AM
JEE main - Chemistry
Asked by Ranjeetgupta26068 | 19 May, 2019, 22:14: PM
JEE main - Chemistry
Asked by g_archanasharma | 20 Feb, 2019, 17:23: PM
JEE main - Chemistry
Asked by g_archanasharma | 08 Feb, 2019, 17:48: PM