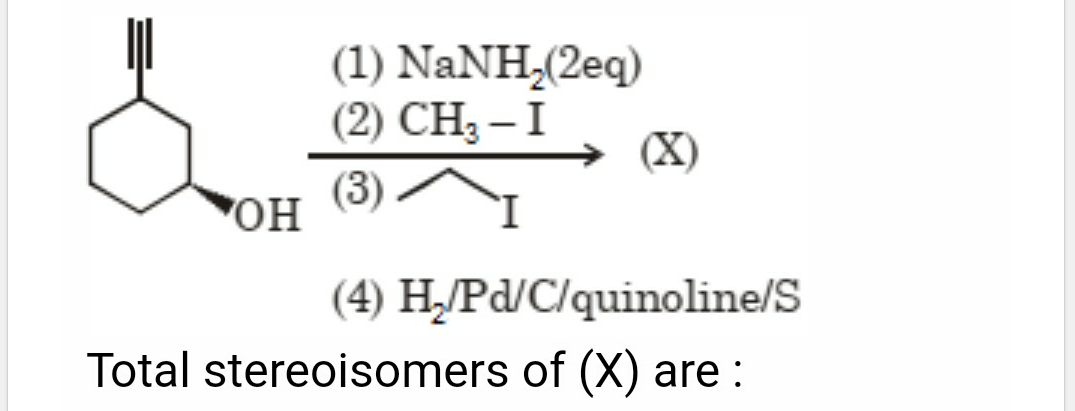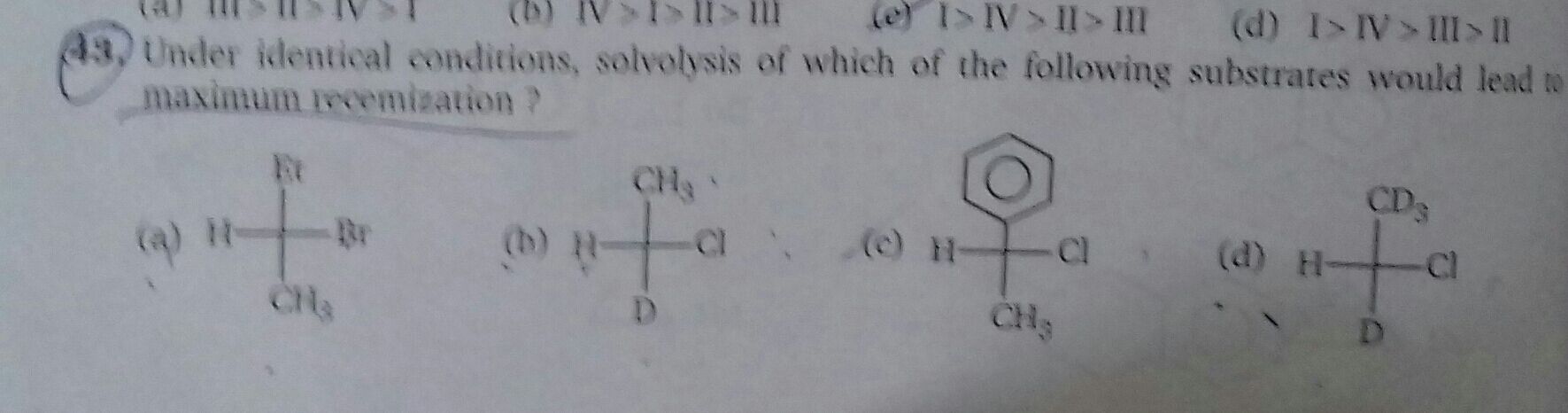JEE Class main Answered
The major product is
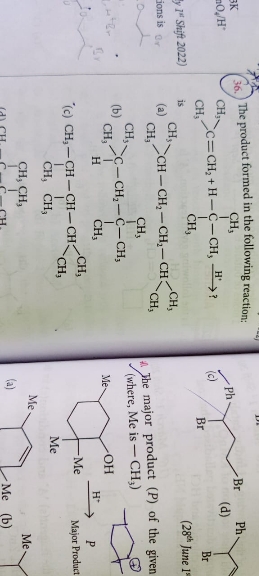
Asked by harshakancharla329 | 27 Jan, 2024, 09:07: AM
Dear Student,
Basis the stability of carbocation and carbanions, the correct answer option is b.
Tertiary carbocation and primary carbanion are the most stable structures.
Hence first carbocation would form by attack of H+ ion on 2-methylprop-1-ene followed by the formation of carbanion by loss of H+ ion from Isobutane. Neative charge due to the lone pair of electrons in carbanion attack positive charge on carbocation which gives structure given in option b.
Answered by | 27 Jan, 2024, 12:14: PM
JEE main - Chemistry
Asked by patelamrutbhaib24 | 28 Jan, 2024, 01:44: PM
JEE main - Chemistry
Asked by harshakancharla329 | 27 Jan, 2024, 09:07: AM
JEE main - Chemistry
Asked by mp797056 | 23 Oct, 2023, 04:23: PM
JEE main - Chemistry
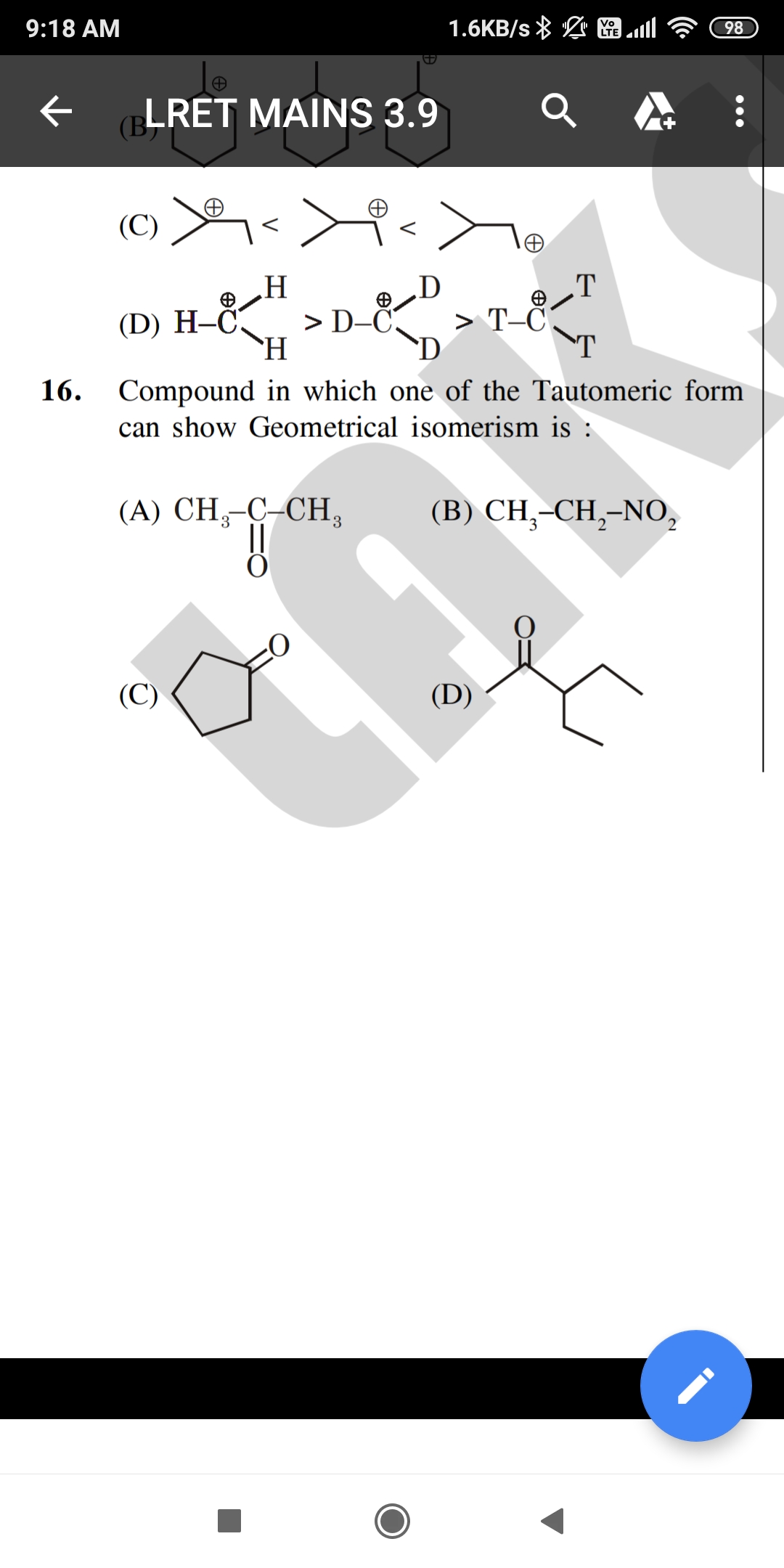
Asked by dheerajrao2005 | 26 Mar, 2022, 07:06: PM
JEE main - Chemistry
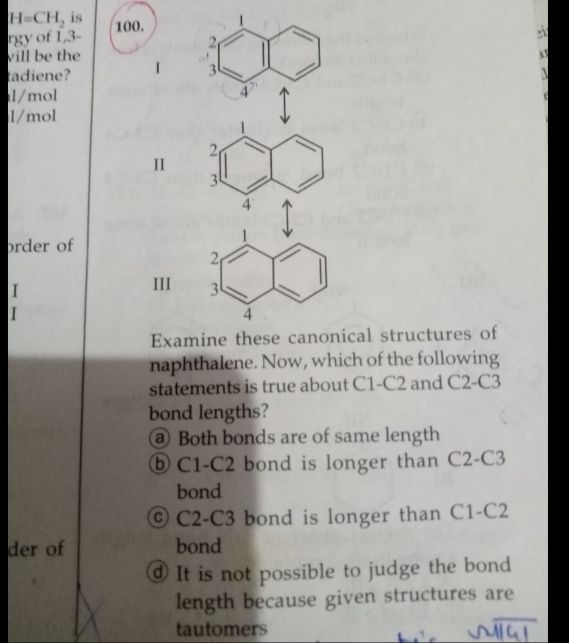
Asked by yasharthshankar | 05 Jun, 2020, 11:39: PM
JEE main - Chemistry
Asked by jhajuhi19 | 28 Mar, 2020, 10:45: AM
JEE main - Chemistry
Asked by bb ki | 10 Feb, 2020, 09:33: PM
JEE main - Chemistry
Asked by ashutosharnold1998 | 29 Dec, 2019, 01:05: PM