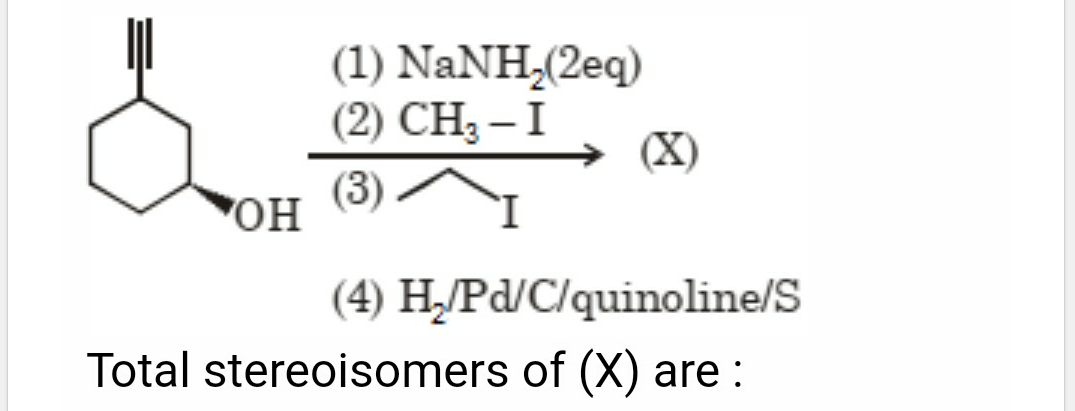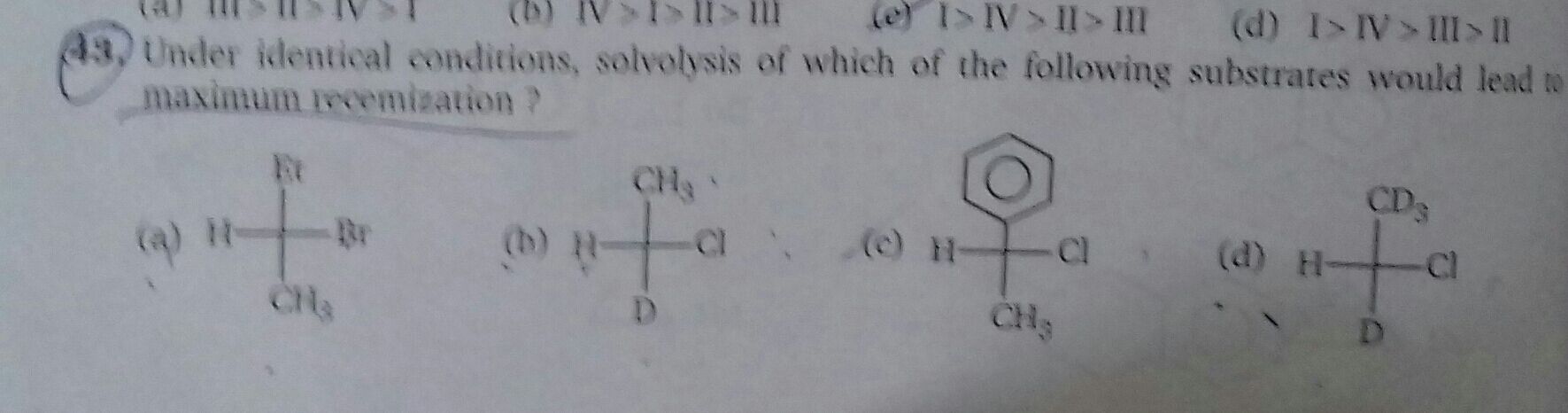JEE Class main Answered
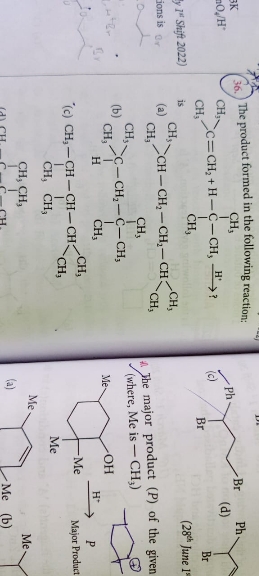
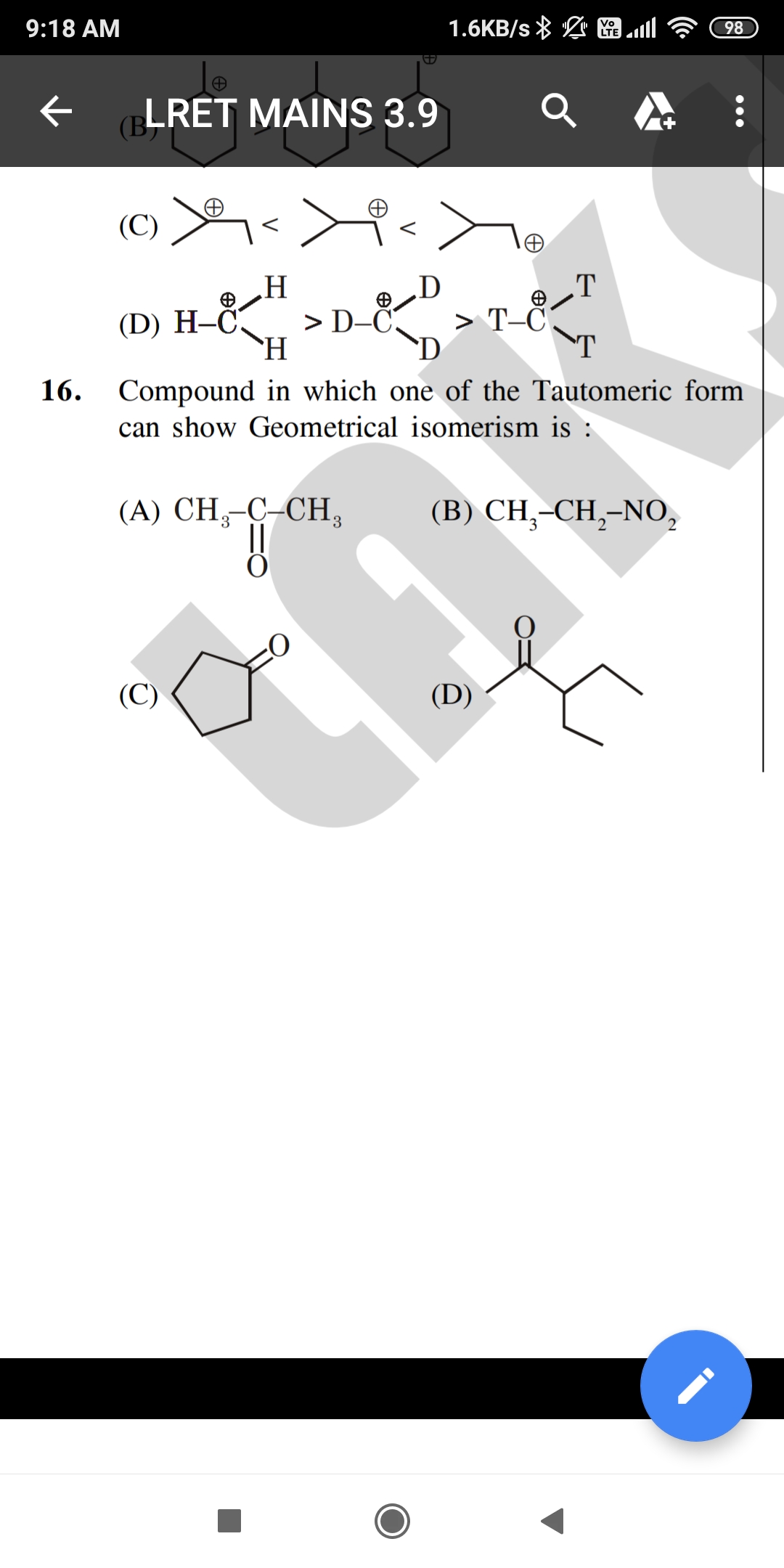
Pick the correct one. Pls explain in detail
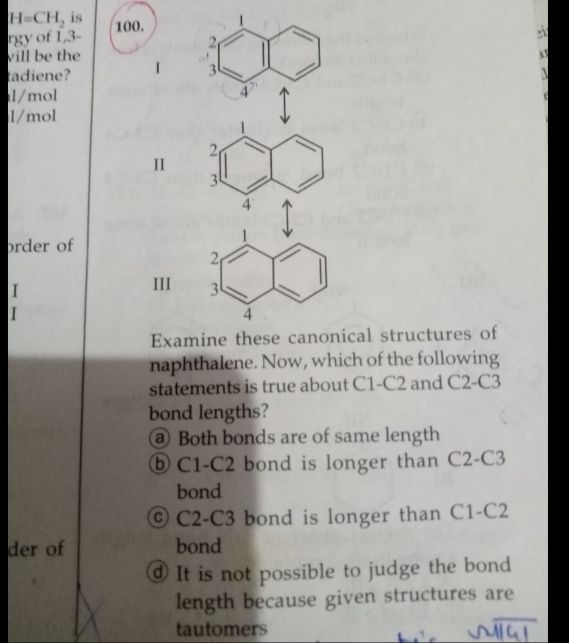
Asked by ashutosharnold1998 | 29 Dec, 2019, 01:05: PM
In these resonance structures, C1-C2 bond is having more double bond character than C2-C3 bond and we know that bond length of double bond is less than single bond.
so Bond length of C1-C2 < Bond length of C2-C3
C2-C3 bond is longer than C1-C2 bond.
Answered by Ravi | 30 Dec, 2019, 03:26: PM
JEE main - Chemistry
Asked by patelamrutbhaib24 | 28 Jan, 2024, 01:44: PM
JEE main - Chemistry
Asked by harshakancharla329 | 27 Jan, 2024, 09:07: AM
JEE main - Chemistry
Asked by mp797056 | 23 Oct, 2023, 04:23: PM
JEE main - Chemistry
Asked by dheerajrao2005 | 26 Mar, 2022, 07:06: PM
JEE main - Chemistry
Asked by yasharthshankar | 05 Jun, 2020, 11:39: PM
JEE main - Chemistry
Asked by jhajuhi19 | 28 Mar, 2020, 10:45: AM
JEE main - Chemistry
Asked by bb ki | 10 Feb, 2020, 09:33: PM
JEE main - Chemistry
Asked by ashutosharnold1998 | 29 Dec, 2019, 01:05: PM