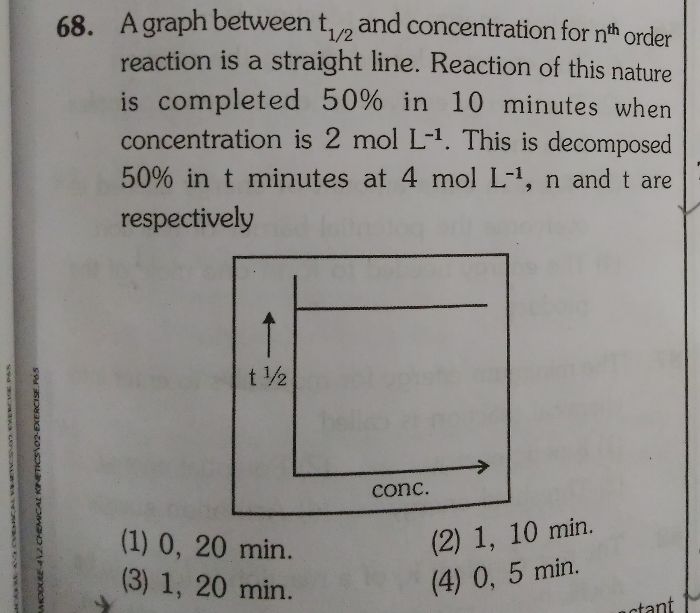
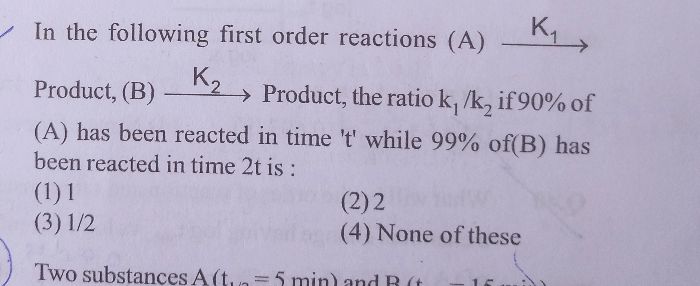CBSE Class 12-science Answered
Dear Student
Nucleophilic Substitution (SN1 and SN2)

Nucleophilic substitution is the reaction of an electron pair donor (the nucleophile, Nu) with an electron pair acceptor (the electrophile). An sp3-hybridized electrophile must have a leaving group (X) in order for the reaction to take place.
Mechanism of Nucleophilic Substitution
The term SN2 means that two molecules are involved in the actual transition state:

The departure of the leaving group occurs simultaneously with the backside attack by the nucleophile. The SN2 reaction thus leads to a predictable configuration of the stereocenter - it proceeds with inversion (reversal of the configuration).
In the SN1 reaction, a planar carbenium ion is formed first, which then reacts further with the nucleophile. Since the nucleophile is free to attack from either side, this reaction is associated with racemization.

In both reactions, the nucleophile competes with the leaving group. Because of this, one must realize what properties a leaving group should have, and what constitutes a good nucleophile. For this reason, it is worthwhile to know which factors will determine whether a reaction follows an SN1 or SN2 pathway.