CBSE Class 11-science Answered
Match the list 1 to list 2

Asked by ashutosharnold1998 | 30 Jan, 2020, 09:07: PM
(a)-iv, (b)-i, (c)-ii, (d)-iii
(a) When KP >Q, the reaction goes in forward reaction, i.e., reaction is spontaneous.
(b) Given 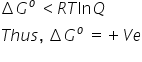
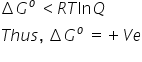
and hence reaction is non-spontaneous.
(c) At equilibrium, KP =Q.
(d) 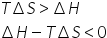
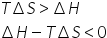
This is condition for endothermic spontaneous reaction.
Answered by Ravi | 31 Jan, 2020, 07:16: PM
Concept Videos
CBSE 11-science - Chemistry
Asked by mankdubey670 | 06 Jun, 2022, 01:27: PM
CBSE 11-science - Chemistry
Asked by gganga | 10 Apr, 2018, 06:02: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 14 Aug, 2014, 04:44: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 15 Jun, 2016, 05:46: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 14 Aug, 2014, 04:51: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 14 Aug, 2014, 04:53: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 14 Aug, 2014, 05:00: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 15 Jun, 2016, 05:46: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 23 Sep, 2014, 03:28: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 23 Sep, 2014, 03:39: PM




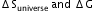 ?
?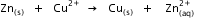 Given that
Given that 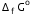 for Cu2+(aq) and Zn2+(aq) as 65 kJ mol-1 and -147.2 kJ mol-1 respectively.
for Cu2+(aq) and Zn2+(aq) as 65 kJ mol-1 and -147.2 kJ mol-1 respectively.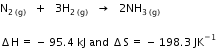 Calculate the temperature at which Gibbs energy change ΔG is equal to zero. Predict the nature of the reaction at this temperature and above it.
Calculate the temperature at which Gibbs energy change ΔG is equal to zero. Predict the nature of the reaction at this temperature and above it.