CBSE Class 12-science Answered
In chromium (III) chloride, CrCl3, the chloride ions have CCP (cubic close packing) arrangement and Cr (III) ions are present in octahedral holes. What fraction of the octahedral holes is occupied? What fraction of the total number of holes is occupied?
Asked by Topperlearning User | 17 Jun, 2016, 10:50: AM
In CCP arrangement each chloride ion would have one octahedral void and two tetrahedral void associated with it.
Number of octahedral voids with 3 chloride ions = 3
Number of tetrahedral voids with 3 chloride ions = 3 x 2 = 6
Total number of voids with 3 chloride ions = 9
Number of octahedral voids occupied by Cr (III) = 1
Fraction of octahedral voids occupied = 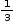
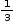
Fraction of total number of voids occupied = 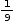
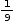
Answered by | 17 Jun, 2016, 12:50: PM
Concept Videos
CBSE 12-science - Chemistry
Asked by shreyasingh3652 | 28 May, 2022, 11:06: PM
CBSE 12-science - Chemistry
Asked by shrinithishri2003 | 22 Jun, 2021, 12:09: AM
CBSE 12-science - Chemistry
Asked by siddikparamban | 01 Apr, 2020, 01:04: PM
CBSE 12-science - Chemistry
Asked by prasadranjan46 | 05 May, 2018, 10:54: AM
CBSE 12-science - Chemistry
Asked by gargimoitreyee | 12 Mar, 2018, 11:51: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 17 Jun, 2016, 10:55: AM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 17 Jun, 2016, 10:57: AM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 17 Jun, 2016, 10:51: AM




