CBSE Class 12-science Answered
In AB2O4, the oxide ions are placed in cubic close packed lattice, cation A is present in tetrahedral voids and cations B are present in octahedral voids. What is the percentage of tetrahedral and octahedral voids occupied by A and B respectively?
Asked by Topperlearning User | 17 Jun, 2016, 10:51: AM
In CCP lattice of oxide ions, there would be two tetrahedral voids and one octahedral void for each oxide ion. Therefore for four oxide ions, there would be 8 tetrahedral and 4 octahedral voids. Out of 8 tetrahedral voids, 1 is occupied by
A and out of 4 octahedral voids 2 are occupied by B
% of tetrahedral voids occupied by A =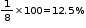
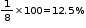
% of octahedral voids occupied by B =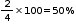
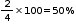
Answered by | 17 Jun, 2016, 12:51: PM
Concept Videos
CBSE 12-science - Chemistry
Asked by shreyasingh3652 | 28 May, 2022, 11:06: PM
CBSE 12-science - Chemistry
Asked by shrinithishri2003 | 22 Jun, 2021, 12:09: AM
CBSE 12-science - Chemistry
Asked by siddikparamban | 01 Apr, 2020, 01:04: PM
CBSE 12-science - Chemistry
Asked by prasadranjan46 | 05 May, 2018, 10:54: AM
CBSE 12-science - Chemistry
Asked by gargimoitreyee | 12 Mar, 2018, 11:51: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 17 Jun, 2016, 10:55: AM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 17 Jun, 2016, 10:57: AM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 17 Jun, 2016, 10:51: AM




