CBSE Class 12-science Answered
cation A occupies 2/3rd of all the octahedral voids while the anion B is found in all lattice points predict the formula of the ionic compound formed by them
Asked by shrinithishri2003 | 22 Jun, 2021, 00:09: AM
Type of crystal lattice is not given.
Let's assume it is hcp.
Coordination number in hcp=12
Cation A is arranged in octahedral voids=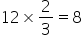
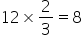
Number of latttice points in hcp=2
Anion B is arranged on lattice points, number of anion B=2
A : B
8 : 2
4 : 1
Formula of compound is A4 B1
Answered by Ravi | 22 Jun, 2021, 10:49: AM
Concept Videos
CBSE 12-science - Chemistry
Asked by shreyasingh3652 | 28 May, 2022, 23:06: PM
CBSE 12-science - Chemistry
Asked by shrinithishri2003 | 22 Jun, 2021, 00:09: AM
CBSE 12-science - Chemistry
Asked by siddikparamban | 01 Apr, 2020, 13:04: PM
CBSE 12-science - Chemistry
Asked by prasadranjan46 | 05 May, 2018, 10:54: AM
CBSE 12-science - Chemistry
Asked by gargimoitreyee | 12 Mar, 2018, 23:51: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 17 Jun, 2016, 10:55: AM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 17 Jun, 2016, 10:57: AM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 17 Jun, 2016, 10:51: AM




