CBSE Class 11-science Answered
From the given compounds which has maximum number of molecules?? (a) 0.1 molar ammonia, (b) 1 gram hydrogen, (c) 40 gram CaCO3, (d) 6.023×10^20 molecules
Asked by patra04011965 | 27 May, 2019, 08:07: AM
We will calculate number of molecules in easch case.
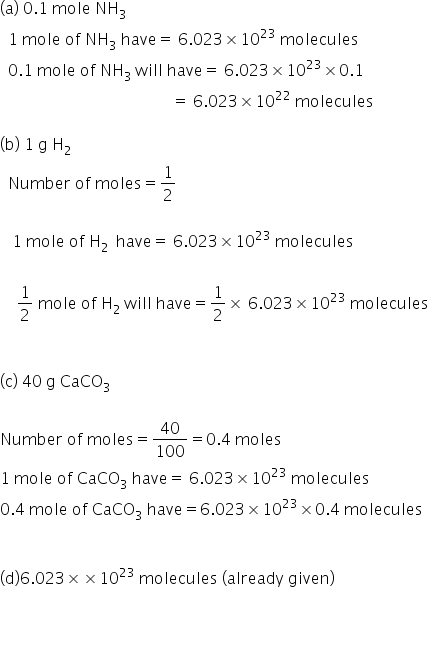
As you can see number of molecules is highest in 1 g H2. So correct answer is (b).
Answered by Ravi | 27 May, 2019, 11:44: AM
Concept Videos
CBSE 11-science - Chemistry
Asked by manikandanragul1 | 11 Apr, 2024, 09:02: AM
CBSE 11-science - Chemistry
Asked by nikhithaguguloth14 | 29 Mar, 2024, 08:15: PM
CBSE 11-science - Chemistry
Asked by sumedhasingh238 | 27 Mar, 2024, 11:04: PM
CBSE 11-science - Chemistry
Asked by avijotsingh946431 | 22 Feb, 2024, 05:36: PM
CBSE 11-science - Chemistry
Asked by gurmelsinghray | 21 Feb, 2024, 08:43: AM
CBSE 11-science - Chemistry
Asked by bablipanwar893 | 01 Jul, 2023, 12:25: PM
CBSE 11-science - Chemistry
Asked by saijagdale9 | 19 Jun, 2023, 02:34: PM
CBSE 11-science - Chemistry
Asked by kdimple765 | 17 Jul, 2022, 01:24: PM
CBSE 11-science - Chemistry
Asked by alfirozislam900 | 03 Jul, 2022, 01:24: PM
CBSE 11-science - Chemistry
Asked by alfirozislam900 | 03 Jul, 2022, 01:23: PM




