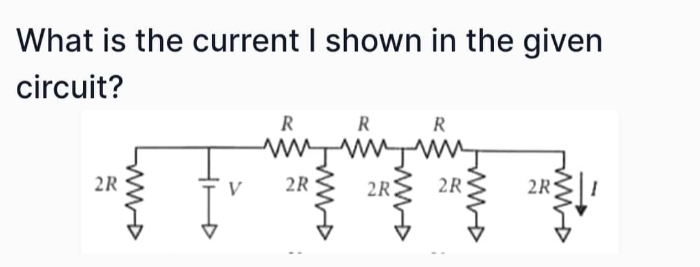
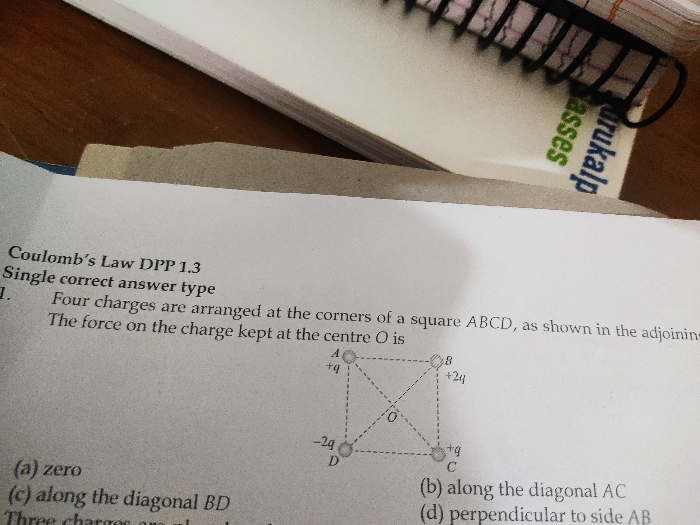JEE Class main Answered
For a given cyclic
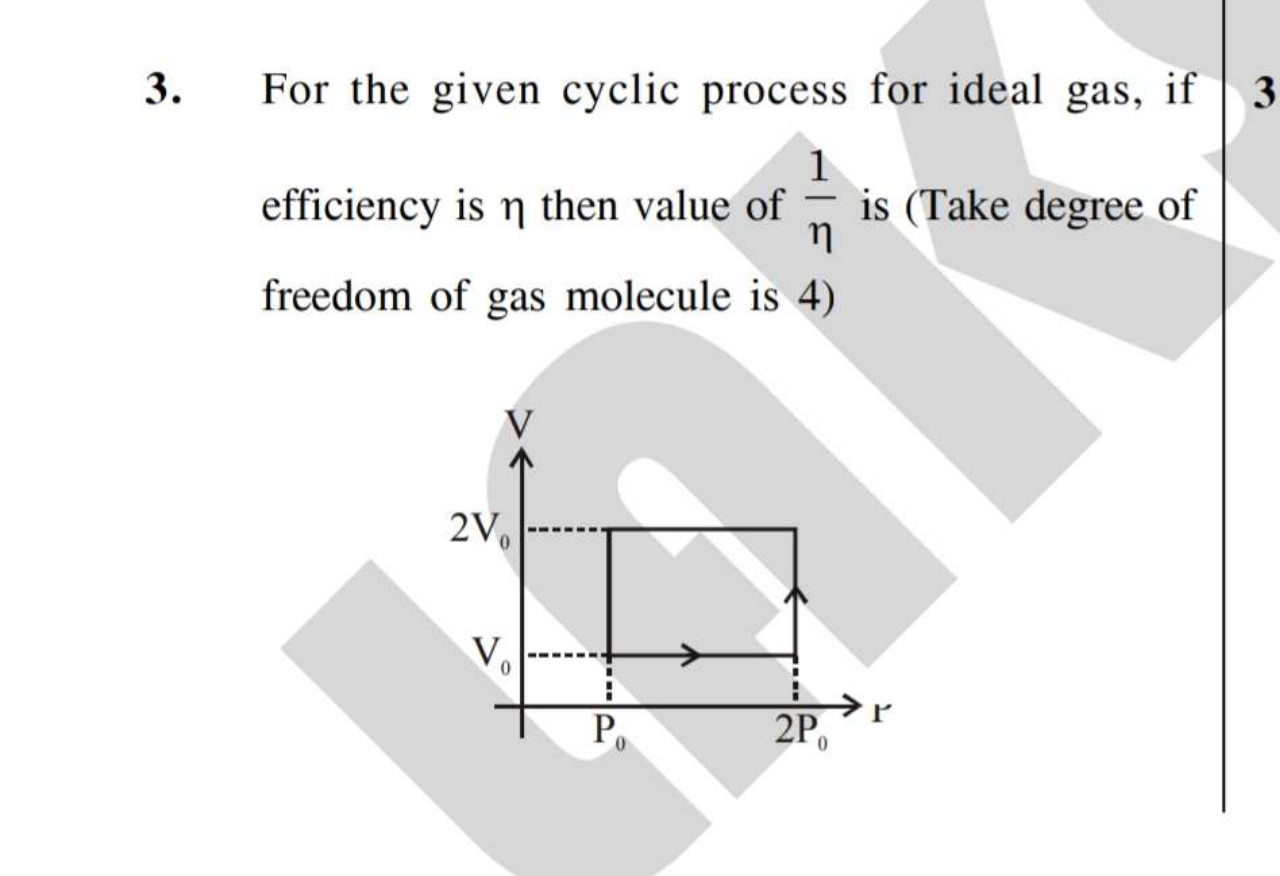
Asked by raj436830 | 22 Mar, 2020, 11:23: AM
Let us get the temperatures of each state.
Let us assume at state-1 , we have the state variables Pressure, volume and temperature are Po , Vo and T respectively.
Process 1-2 is at constant volume. Pressure is proportional to temperature for ideal gas at constant volume.
hence , we have, ( Po / T ) = ( 2 Po / T2 ) .......................(1)
where T2 is temperature at state 2 . Hence from above equation, T2 = 2T
Process 2-3 is at constant pressure. Volume is proportional to temperature for ideal gas at constant pressure.
hence , we have, ( Vo / 2T ) = ( 2Vo / T3 ) .......................(2)
where T3 is temperature at state 3 . Hence from above equation, T3 = 4T
Process 3-4 is at constant volume. Pressure is proportional to temperature for ideal gas at constant volume.
hence , we have, ( 2Po / 4T ) = ( 2 Po / T4 ) .......................(3)
where T4 is temperature at state 4 . Hence from above equation, T4 = 2T
Workdone for process 1-2 , W12 = 0 ( constant volume , dV = 0 ) ,
Heat transfer for process 1-2, Q12 = n Cv ΔT = n (2R)( 2T - T) = 2nRT
{ Cv = no. of degrees of freedom × (R/2) }
Workdone for process 2-3 , W23 = 2Po ( 2Vo - Vo) = 2 PoVo = 2nRT
{ Cv = [ no. of degrees of freedom × (R/2) + R ] }
Heat transfer for process 2-3, Q23 = n Cp ΔT = n (3R)( 4T - 2T) = 6nRT
Workdone for process 3-4 , W34 = 0 ( constant volume , dV = 0 ) ,
Heat transfer for process 3-4, Q34 = n Cv ΔT = n (2R)( 2T - 4T) = - 4nRT
Workdone for process 4-1 , W41 = Po ( Vo - 2Vo) = - PoVo = - nRT
Heat transfer for process4-1, Q41 = n Cv ΔT = n (3R)( T - 2T) = -3nRT
Net workdone = W12 + W23 + W34 + W41 = 2nRT - nRT = nRT
Heat absorbed by system = Q12 +Q23 = 8nRT
Efficiency η = Net workdone / Absorbed Heat = (nRT) / (8nRT) = 1/8
Hence 1/η = 8
Answered by Thiyagarajan K | 22 Mar, 2020, 02:56: PM
Application Videos
JEE main - Physics
Asked by arivaryakashyap | 23 Apr, 2024, 10:40: AM
JEE main - Physics
Asked by ratnadeep.dmr003 | 21 Apr, 2024, 11:06: PM
JEE main - Physics
Asked by ksahu8511 | 19 Apr, 2024, 11:55: AM
JEE main - Physics
Asked by mohammedimroz | 13 Apr, 2024, 09:48: PM
JEE main - Physics
Asked by medhamahesh007 | 02 Apr, 2024, 11:11: AM
JEE main - Physics
Asked by gundlasumathi93 | 31 Mar, 2024, 02:13: PM
JEE main - Physics
Asked by chhayasharma9494 | 31 Mar, 2024, 12:47: PM
JEE main - Physics
Asked by archithateja3 | 30 Mar, 2024, 10:23: PM
JEE main - Physics
Asked by Machinenineha | 27 Mar, 2024, 05:28: PM