Boiling And Freezing Points Of Solutions Free Doubts and Solutions
CBSE - XII Science - Chemistry - Solutions
all question

CBSE - XII Science - Chemistry - Solutions
The boiling point of benzene is 353.23k. When 1.80g of a non-volatile solute was dissolved in 90g of benzene , the boiling is raised to 345.11k. Calculate the molar mass of solute (kb=2.53kg mol-1)
CBSE - XII Science - Chemistry - Solutions
Molal elevation constant for benzene is 2.5K/m. A solution of some organic substance in benzene boils at 0.126°C higher than benzene. What is the molality of solution?
CBSE - XII Science - Chemistry - Solutions
Boiling point of water at 750mm of hg is 99.63°C. how much sucrose is to be added to 500g of water such that it boils at 100°C.
CBSE - XII Science - Chemistry - Solutions
2.00g of non-electolyte solute dissolved in 100g of benzene, lowered the freezing point of benzene by 0.30K. find the molar mass of solute.
CBSE - XII Science - Chemistry - Solutions
What is colligative property
CBSE - XII Science - Chemistry - Solutions
Explain B.pt.elevation and F.pt.depression?
CBSE - XII Science - Chemistry - Solutions
To 500 cm3of water ,3×10^-3 of acetic acid is added .if 23 percent is dissociated what will be the depression in freezing pt given k1 is 1.86K kg /mol
CBSE - XII Science - Chemistry - Solutions
two liquids a and b at 120C and 160C which of these liquids has at higher vapour pressure at 90C
CBSE - XII Science - Chemistry - Solutions
calculate freezing point of a solution when 54g of glucose is dissolved in 250g of water
CBSE - XII Science - Chemistry - Solutions
Calculate the amount of CaCl2, which must be added to 2 kg water so that the freezing point is depressed by 61 K (Kf of H2O =1.86 K kg mol^-1, Atomic mass of Ca = 40, Cl = 35.5)
CBSE - XII Science - Chemistry - Solutions
please answer this

CBSE - XII Science - Chemistry - Solutions
12gram glucose ko 10 gm solution Ka kwathnank 100.34 c hai,molal unnayan sthirank batao
CBSE - XII Science - Chemistry - Solutions
Calculate freezing point lowering of a 5% solution of glucose in water
CBSE - XII Science - Chemistry - Solutions
Depression in freezing point is 6 K for NaCl solution if kf for water is 1.86 K/kg mol, amount of NaCl dissolved in 1 kg water is (a)3.42 (b)1.62 (c)3.24 (d)1.71
CBSE - XII Science - Chemistry - Solutions
Why ice cream seller add salt to ice around cones and not ice alon why ? I have searched a lot about the reason but I can't understand any reason? Why adding of salt makes water colder
CBSE - XII Science - Chemistry - Solutions
Calculate the boiling point of a solution containing 0.45g of camphor (mol. wt. 152) dissolved in 35.4g of acetone (b.p. 56.3°C); Kb per 100 gm of acetone is 17.2°C. IN THIS QUESTION WE USE THE FORMULA = 100 Kb *w2/w1M2 I want to know how we derive this formula in which 100 is in numerator and not 1000 . I know Kb is per 100 g though I am not able to derive this relation
CBSE - XII Science - Chemistry - Solutions
Please explain the paragraph given in pic How 100 is written in numerator
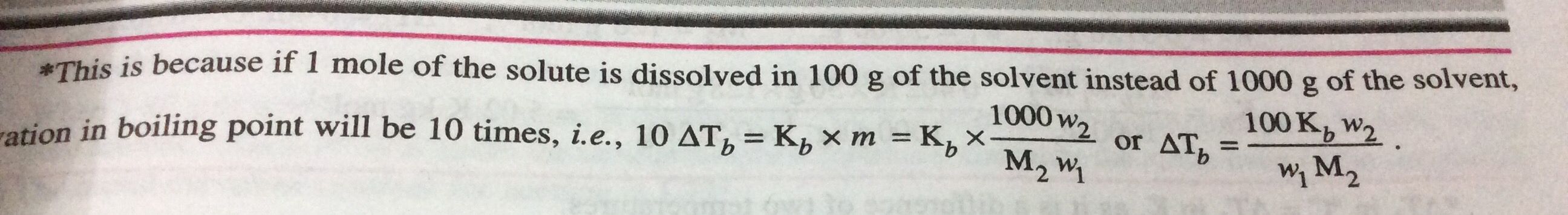
CBSE - XII Science - Chemistry - Solutions
Please explain that what does it mean in the paragraph in the pic given and also explain that what it means that " the solution boils at a constant temperature and composition remains same"

CBSE - XII Science - Chemistry - Solutions
when0.5g Nacl present in 1000g of water then calculate the freezing point of solution ? kf= 1.7k kg /mol
CBSE - XII Science - Chemistry - Solutions
1.00 g of a non-electrolyte solute dissolved in 50 g of benzene lowered the freezing point of benzene by 0.40K. The freezing point depression constant of benzene is 5.12 K kg/mol. Find the molar mass of the solute
CBSE - XII Science - Chemistry - Solutions
18g glucose is added to 178.2g water the vapour pressure of water for this aqueous solution at 100calsias is
CBSE - XII Science - Chemistry - Solutions
HI SIR GOOD MORNING....SIR I HAVE A DOUBT IN ELEVATION OF BOILING POINT.....
CBSE - XII Science - Chemistry - Solutions
The mole fractions of two components X and Y were found to be same in a binary solution. Will their mole fractions in vapour phase be the same or different?
CBSE - XII Science - Chemistry - Solutions
How many grams of KCL should be added to 1 kg of water to lower its boiling point to -8 degree celsius(kf of water=1.86)?
CBSE - XII Science - Chemistry - Solutions
Density of 1M of a non-electrolyte aqueous solute of molecular weight 100 is 2 g/mL. If Kf(H2O) is 1.86°C kg mol–1, then solution will freeze at
CBSE - XII Science - Chemistry - Solutions
Calculate molal elevation constant of water given that 0•1molal aq solution of a substance boiled at 100•052°C
CBSE - XII Science - Chemistry - Solutions
How COLLIGATIVE PROPERTIES are useful in measuring molecular weight ?
CBSE - XII Science - Chemistry - Solutions
A SOLUTION OF 1.25g OF NON ELECTROLYTE IN 20g OF WATERFREEZESAT 271.94K.IF Kf=1.86 THEN MOLECULAR WEIGHT OF SOLUTION WILL BE..?????
CBSE - XII Science - Chemistry - Solutions
Calculate the freezing point of a solution containing 60 g of glucose (Molar mass = 180g mol-1) in 250 g of water. (Kf of water = 1.86 K kg mol-1)
CBSE - XII Science - Chemistry - Solutions
Please give answer of the following

CBSE - XII Science - Chemistry - Solutions
A solution X is prepared by mixing ethanol and water. The mole fraction of ethanol in the mixture is 0.8, then depression in freezing point of solution X is. ( Kf water = 1.86 and Kf ethanol = 2.0 )
1. 4.34 K
2. 10.86 K
3. 5.434 K
4. 1.35 K
CBSE - XII Science - Chemistry - Solutions
find how many grams of KCL should be added to 1 kg of water to lower its freezing point to -8 degree celsius( kf =1.86 K Kg/ mol) pls explain each and every step
CBSE - XII Science - Chemistry - Solutions
what would be the freezing point of aq. sol. containg 17 g of C2H5OH in 1000g of water Kf=1.86Kmolality^-
CBSE - XII Science - Chemistry - Solutions
Q. the molal freezing point constant of watr is 1.86 K molality ^-1 if 342 g of can sugar (C6H22O11) are dissolved in 1000g water the solution will freezze at
Q. molal depression of freezing point of water is 1.86 degree per 1000g water . 0002 mole of urea dissolved in 100g of water will produce a lowering of temp. of
CBSE - XII Science - Chemistry - Solutions
the freezing pointof a 0.05 molal solution of a non electrolyte in water is
CBSE - XII Science - Chemistry - Solutions
Calculate the temperature at which a solution containing 54 g of glucose, (C6 H12 O6), in 250 g of water will freeze. (Kf for water = 1.86 K mol-1 kg)
CBSE - XII Science - Chemistry - Solutions
What is molal elevation constant? What are its units?
CBSE - XII Science - Chemistry - Solutions
Plz explain me in detail how with addition of a non-volatile solute depression in freezing point occurs alongwith its graph. plz explain this using concept of variation in vapour pressure and temperature.
CBSE - XII Science - Chemistry - Solutions
pls sir explain OSTWALD WALKER DYNAMIC METHOD in details?
CBSE - XII Science - Chemistry - Solutions
show that relative lowering of vapour pressure is a colligative property.( for 3 marks)
CBSE - XII Science - Chemistry - Solutions
What is the mass of iodine to be dissolved in 100g of benzene to obtain a solution that freezes at 2.94 degree C ? Freezing point of benzene is 5.5 degree C and Kf=5.12Km-1
CBSE - XII Science - Chemistry - Solutions
the molar volume of liquid benzene(density=0.867gm/ml)increased by a factor of 2750 at 20 degree celsius and that of liquid toluene(density=0.867gm/ml)increases by a factor of 7720 at 20 degree celsius . a solution of benzene and toluene at 20 degree celsius has a vapour pressure of 46 torr . find the mole fraction of benzene in the vapour above the solution.
CBSE - XII Science - Chemistry - Solutions
100g of a non-electrolyte solute is dissolved is 50g of benzene lowers the freezing point of benzene by 0.4K. find the molar mass of solute. Kf for benzene is 5.12Kkg/mol.
100g of a non-electrolyte solute is dissolved is 50g of benzene lowers the freezing point of benzene by 0.4K. find the molar mass of solute. Kf for benzene is 5.12Kkg/mol.



