Association And Dissociation Free Doubts and Solutions
CBSE - XII Science - Chemistry - Solutions
The formula of degree of dissociation
CBSE - XII Science - Chemistry - Solutions
HOW TO FIND VANT OF FACTOR
CBSE - XII Science - Chemistry - Solutions
Value of Van't Hoff factor if CH3COOH 60% dissociates and 40% dimerized is–(1) 1.2, (2) 1.4, (3) 1.6, (4) 1.8
CBSE - XII Science - Chemistry - Solutions
I know that answer is (a) but please explain the reason in detail
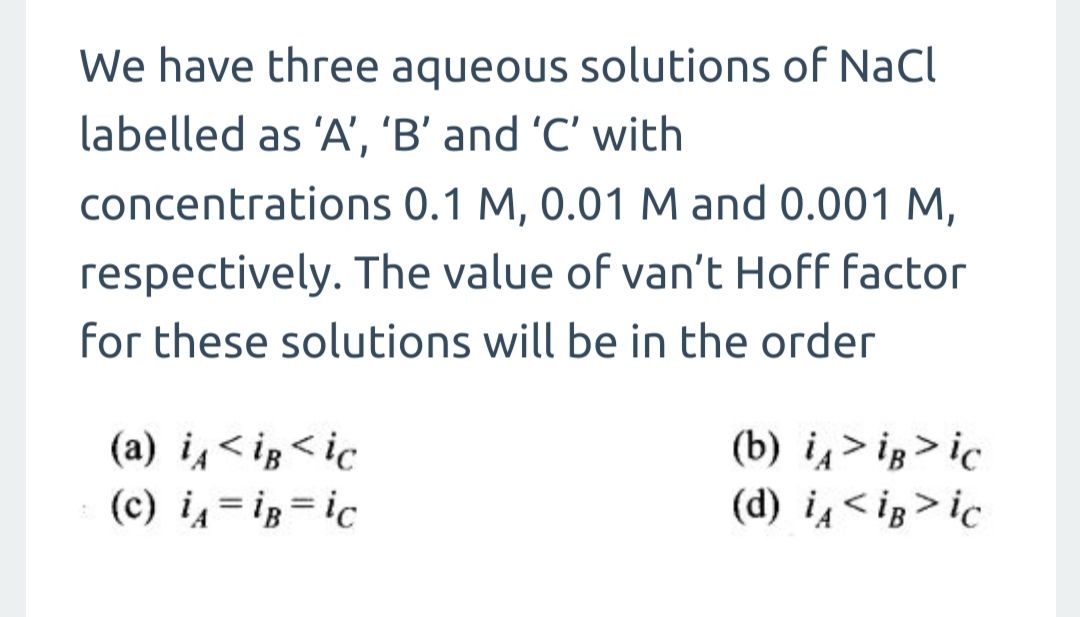
CBSE - XII Science - Chemistry - Solutions
We say in a solution made up of a solvent and a solute, the majour component is solvent and the sbstance present in small propotions is solute.
But if we take the case of a 50-50 solution where each component is present at equaly, how can we diffrentiate between a solvent and a solute?
CBSE - XII Science - Chemistry - Solutions
why is the i value of sugar and glucose 1
CBSE - XII Science - Chemistry - Solutions
when to use R=8.314 and R=0.008314
CBSE - XII Science - Chemistry - Solutions
Predict the Boiling point of solution prepared by dissolving 25.0g of urea and 25.0 g of thiourea in 100 gram of water. Given for water Kb= 0.52 K Kg mol-1 and Boiling point of pure water is 373.15 K.
CBSE - XII Science - Chemistry - Solutions
0.216molal solution of Cadmium sulphate is prepared in 1000gram of water. The depression in freezing point was measured to be 0.284K. Calculate the Vant hoff factor. The Cryoscopic constant for water is 1.86K Kg mol-1.
CBSE - XII Science - Chemistry - Solutions
What does the line, ' Depending on the vapour pressures of the pure components 1 and 2, total vapour pressure over the solution decreases or increases with the increase of the mole fraction of component 1' ?
CBSE - XII Science - Chemistry - Solutions
I got a project on chemistry where I have to do an experiment at home. Please suggest me a topic keeping in mind that the required materials can be easily found that can be done at home. Please dont confuse with the name of the chapter and the topic name that Ihave given. It was compulsory to fill those up.
CBSE - XII Science - Chemistry - Solutions
how to find equivalent weight?
CBSE - XII Science - Chemistry - Solutions
A 0.1 molar NaCl soln. exerts an osmotic pressure of 4.82 atm at 27 degree of dissociation of Nacl.vol=10l w=58.5
CBSE - XII Science - Chemistry - Solutions
Consider separate solutions of 0.5 M CH3OH, 0.250 M KCl (aq) and 0.125 M Na3PO4
- (aq). Arrange the above solutions in the increasing order of their Van’t Hoff factor.Give reason also ...
- (aq). Arrange the above solutions in the increasing order of their Van’t Hoff factor.Give reason also ...


