Ideal Solutions Free Doubts and Solutions
CBSE - XII Science - Chemistry - Solutions
question

CBSE - XII Science - Chemistry - Solutions
what is roults law
CBSE - XII Science - Chemistry - Solutions
what is hetrogeneaus
CBSE - XII Science - Chemistry - Solutions
how this question can be answered

CBSE - XII Science - Chemistry - Solutions
(p= kh*x) x is mole fraction.we have to find solubility that is molarity..so how to find molarity

CBSE - XII Science - Chemistry - Solutions
relative lower vapour pressure
CBSE - XII Science - Chemistry - Solutions
please answer this
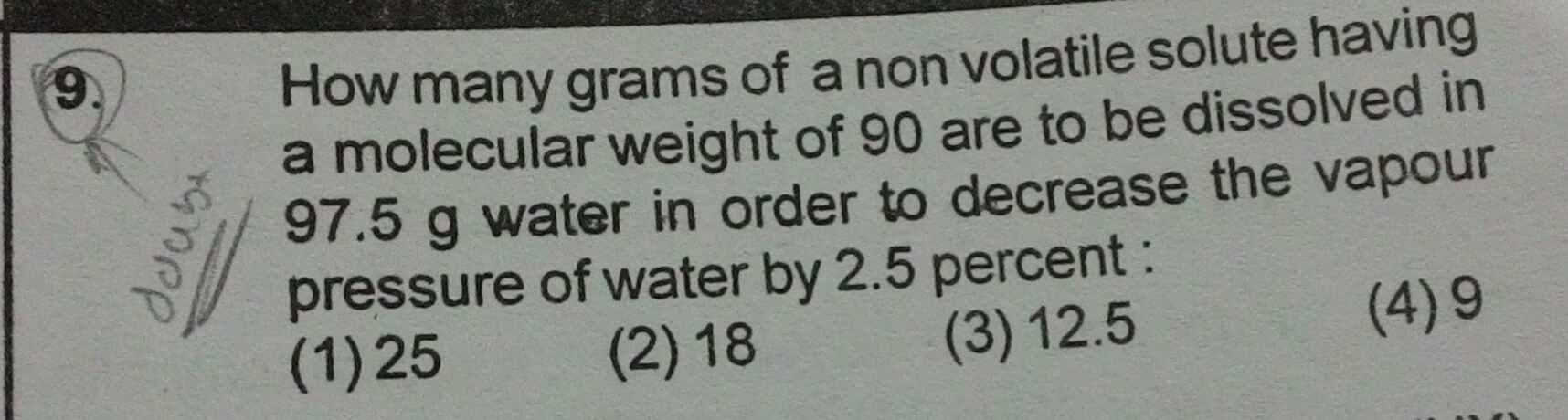
CBSE - XII Science - Chemistry - Solutions
please answer this

CBSE - XII Science - Chemistry - Solutions
pentane and heptene have a vapour pressure of 55pa and 4.8 respectively at 20oc. A mixture is known to contahoeptin 2.52g of pentane and 1400g of heptene.calculate a.the mole fraction of each components in the liquid b.the total vapour pressure of the mixture. c.the mole fraction of each component of the vapour phase
CBSE - XII Science - Chemistry - Solutions
What type of sol is formed on mix equal volume of n hexane or n heptane or bromoethane and chloroethane or benvjene or toulen
CBSE - XII Science - Chemistry - Solutions
In Pradeep publication the definition of solution is " it is a homogenous mixture of two or more chemically non reactive substances" IS THIS DEFINITION CORRECT?
CBSE - XII Science - Chemistry - Solutions
Ans with explanation please

CBSE - XII Science - Chemistry - Solutions
Please can you help me by explaining the answer in detail...
 Asked by nitishkrnehu09
1st January 2018,
3:55 AM
Asked by nitishkrnehu09
1st January 2018,
3:55 AM
CBSE - XII Science - Chemistry - Solutions
an ideal solution containing 1mol each of A and B is vapourised and condensed. The condensate is again vapourised and then condensed.This process is repeated 1000 times.Then find mole fraction of A and B in final liquid solution
CBSE - XII Science - Chemistry - Solutions
State Henry's law correlating the pressure of a gas and its solubility in a solvent and mention two applications for the law.
CBSE - XII Science - Chemistry - Solutions
The vapor pressure of ethanol at 296K is 40 mm of Hg, its mole fraction in a solution having methanol is 0.8.Calculate the vapor pressure of resultant solution.(assume methanol to be non volatile).
CBSE - XII Science - Chemistry - Solutions
The vapor pressure of A at 296K is 40 mm of Hg, its mole fraction in a solution having methanol is 0.5.Vapour pressure of other component B is 25.0mm Hg. Calculate the vapor pressure of resultant solution.
CBSE - XII Science - Chemistry - Solutions
Vapor pressure of ethanol and Methanol are 45 and 90mm Hg at a given temperature. If 60gm of ethanol and 40gm of methanol are mixed to form an ideal solution, calculate the vapour pressure of solution at the same temperature.
CBSE - XII Science - Chemistry - Solutions
A saturated solution of hydrogen sulfide in water can be prepared by bubbling H2S gas into water until no more dissolves. Calculate the molality of this solution if 0.385 grams of H2S gas dissolve in 100 grams of water at 20oC and 1 atm.
CBSE - XII Science - Chemistry - Solutions
11.11g of urea is dissolved in 100g of water. What will be the molality of the solution
11.11g of urea is dissolved in 100g of water. What will be the molality of the solution
CBSE - XII Science - Chemistry - Solutions
A solution of NaOH is prepared by dissolving 1.6g of NaOH in 500cm^3 of water. Calculate molarity of the solution.
A solution of NaOH is prepared by dissolving 1.6g of NaOH in 500cm^3 of water. Calculate molarity of the solution.


