CBSE Class 12-science Answered
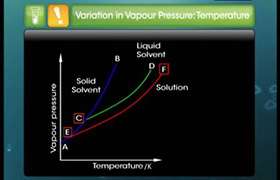
Please explain that what does it mean in the paragraph in the pic given and also explain that what it means that " the solution boils at a constant temperature and composition remains same"

Asked by govtsecschoolnayaganv051 | 20 May, 2019, 01:57: PM
Liquid will begin to boil when
Atmospheric pressure=Vapour pressure
When boiling occurs, molecules absorbs energy and change to a gas, spread out and form bubbles. These rise to the surface and enter the atmosphere.It requires energy to change form liquid to gas. Gas molecules take away energy from liquid. so boiling temperature does not change.
Composition does not channge which means solution is a uniform mixture so after boiling consituents distributed uniformly
Answered by Ravi | 22 May, 2019, 06:51: PM
Concept Videos
CBSE 12-science - Chemistry
Asked by varinder2149 | 10 Dec, 2023, 08:21: PM
CBSE 12-science - Chemistry
Asked by vekariyaparth61 | 16 May, 2022, 04:33: PM
CBSE 12-science - Chemistry
Asked by goyalpavitarta | 03 May, 2021, 01:27: PM
CBSE 12-science - Chemistry
Asked by goyalpavitarta | 03 May, 2021, 01:25: PM
CBSE 12-science - Chemistry
Asked by goyalpavitarta | 03 May, 2021, 01:20: PM
CBSE 12-science - Chemistry
Asked by sshashu993 | 25 Jul, 2020, 08:02: AM
CBSE 12-science - Chemistry
Asked by anukritisingh8103.bmps | 15 Jul, 2020, 05:52: PM
CBSE 12-science - Chemistry
Asked by sharmasherryal | 25 May, 2020, 09:54: AM
CBSE 12-science - Chemistry
Asked by panthpreet0221 | 06 May, 2020, 10:41: AM