CBSE Class 12-science Answered
18g glucose is added to 178.2g water the vapour pressure of water for this aqueous solution at 100calsias is
Asked by araj02582 | 08 Apr, 2019, 12:56: PM
Given:
Weight of glucose = 18 g
Vapour pressure of water = 760 torr
Weight of water = 178.2 g
Moles of glucose =
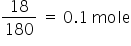
Moles of water =
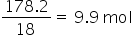
Total no. of moles = 0.1 + 9.9
= 10 mol
Mole fraction of glucose = Change in pressure(ΔP) with respect to initial pressure (P°)

Vapour pressure of the solution is 752.4 torr.
Answered by Varsha | 08 Apr, 2019, 04:50: PM
Concept Videos
CBSE 12-science - Chemistry
Asked by varinder2149 | 10 Dec, 2023, 08:21: PM
CBSE 12-science - Chemistry
Asked by vekariyaparth61 | 16 May, 2022, 04:33: PM
CBSE 12-science - Chemistry
Asked by goyalpavitarta | 03 May, 2021, 01:27: PM
CBSE 12-science - Chemistry
Asked by goyalpavitarta | 03 May, 2021, 01:25: PM
CBSE 12-science - Chemistry
Asked by goyalpavitarta | 03 May, 2021, 01:20: PM
CBSE 12-science - Chemistry
Asked by sshashu993 | 25 Jul, 2020, 08:02: AM
CBSE 12-science - Chemistry
Asked by anukritisingh8103.bmps | 15 Jul, 2020, 05:52: PM
CBSE 12-science - Chemistry
Asked by sharmasherryal | 25 May, 2020, 09:54: AM
CBSE 12-science - Chemistry
Asked by panthpreet0221 | 06 May, 2020, 10:41: AM