NEET Class neet Answered
By dissolving 5g substance in 50g of water, the decrease in freezing point is 1.2 degree celcius. The gram molal depression is 1.85 degree celcius. The molecular weight of substance is
(a)105.4
(b)118.2
(c)137.2
(d)154.2
Asked by Balbir | 28 Jul, 2019, 06:08: PM
Option (d) is correct.
Given:
ΔTf = 1.2 ºC
Wsolvent = 50 gm
Wsolute = 5 gm
Kf = 1.85 ºC gm/mol
Using the formula od depression in freezing point, molecular weight of the subastance can be calculated as,
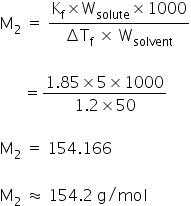
Molecular weight of the subastance is 154.2 g/mol
Answered by Varsha | 29 Jul, 2019, 09:51: AM
Concept Videos
NEET neet - Chemistry
Asked by 8239682116rahul | 10 Apr, 2024, 01:48: PM
NEET neet - Chemistry
Asked by ramadevisupriya5678 | 28 Mar, 2024, 02:18: PM
NEET neet - Chemistry
Asked by myindiaisbad | 17 Jun, 2022, 11:17: AM
NEET neet - Chemistry
Asked by bhaveshkaria31 | 30 May, 2022, 09:26: PM
NEET neet - Chemistry
Asked by rautganesh2255 | 01 Jul, 2021, 09:32: AM
NEET neet - Chemistry
Asked by NituBarman192 | 01 Jun, 2021, 10:22: PM
NEET neet - Chemistry
Asked by bhagirathdangi12345 | 12 Feb, 2021, 01:42: PM
NEET neet - Chemistry
Asked by akashmanu09 | 08 Jan, 2021, 10:21: AM
NEET neet - Chemistry
Asked by arnavvidudala20050 | 17 May, 2020, 03:07: PM