JEE Class main Answered
answer
Asked by azmi13165 | 25 Feb, 2024, 11:27: AM
Dear Student,
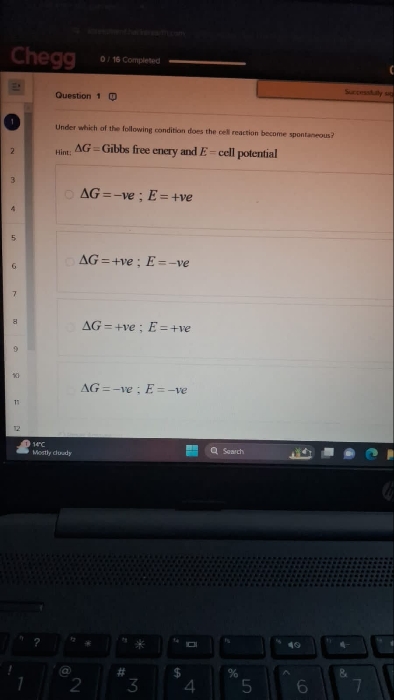
the reaction becomes spontaneous when Gibbs free energy is negative and cell potential is positive.
Hence, the correct answer option is ΔG = -ve and E = +ve.
Answered by | 25 Feb, 2024, 06:46: PM
JEE main - Chemistry
Asked by ashutosharnold1998 | 03 Nov, 2019, 08:22: PM
JEE main - Chemistry
Asked by ashutosharnold1998 | 31 Oct, 2019, 07:16: PM
JEE main - Chemistry
Asked by ashutosharnold1998 | 10 Aug, 2019, 12:10: AM
JEE main - Chemistry
Asked by Ranjeetgupta26068 | 19 May, 2019, 10:14: PM
JEE main - Chemistry
Asked by g_archanasharma | 20 Feb, 2019, 05:23: PM
JEE main - Chemistry
Asked by g_archanasharma | 08 Feb, 2019, 05:48: PM