CBSE Class 9 Answered
(a) A solution is a homogenous mixture of two or more than two substances for e.g. solution of sugar in water.
(b) Properties of solution are:
1. A solution is a homogeneous mixture .
2. The particles of a solution are smaller than 1 nm (10-9 metre) in diameter. So, they cannot be seen by naked eyes.
3. The particles of solution do not scatter a beam of light passing through the solution.
4. The solute particles cannot be separated from the mixture by the process of filtration.
(b) Concentration of a solution is the amount of solute present in a given amount of solution.
Formula:
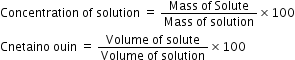
Or
Characteristics :-
|
|
|
True solution |
Suspension |
Colloidal solution |
|
(a) |
Appearance |
Clear and transparent |
Opaque |
Translucent |
|
(b) |
Visibility |
Particles are not visible |
Particles are visible to naked eyes |
Particles are visible under only electron microscope |
|
(c) |
Filterability |
Can pass through the filter paper and animal membrane |
Do not pas through filter paper and animal membrane |
Can pass through filter paper but not through animal membrane |
|
(d) |
Tyndall effect |
Do not show Tyndall effect |
Show Tyndall effect |
Show Tyndall effect |
|
(e) |
Particle size |
Less than 1nm |
More than 100 nm |
Between 1nm and 100 nm |









