CBSE Class 12-science Answered
A FCC lattice cube is formed by atoms A and B. If atom A is present at the corner of the cube and the atom B at the faces of the cube. Find out the formula of the compound?
Asked by Topperlearning User | 17 Jun, 2016, 10:55: AM
Contribution of atom A at eight corners of the cube = 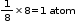
Contribution of atom at each face = 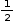 atom
atom
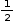 atom
atomContribution of atom B at six faces of the cube = 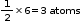
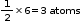
Formula of the compound = AB3
Answered by | 17 Jun, 2016, 12:55: PM
Concept Videos
CBSE 12-science - Chemistry
Asked by shreyasingh3652 | 28 May, 2022, 11:06: PM
CBSE 12-science - Chemistry
Asked by shrinithishri2003 | 22 Jun, 2021, 12:09: AM
CBSE 12-science - Chemistry
Asked by siddikparamban | 01 Apr, 2020, 01:04: PM
CBSE 12-science - Chemistry
Asked by prasadranjan46 | 05 May, 2018, 10:54: AM
CBSE 12-science - Chemistry
Asked by gargimoitreyee | 12 Mar, 2018, 11:51: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 17 Jun, 2016, 10:55: AM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 17 Jun, 2016, 10:57: AM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 17 Jun, 2016, 10:51: AM




